
RETURN to Periodic Table
Silicon is the 14th element on the periodic table. It has 14 protons and 14 neutrons in the nucleus, giving it a mass of 28 amu, and it has 14 electrons enveloping the nucleus.
Electron Shell
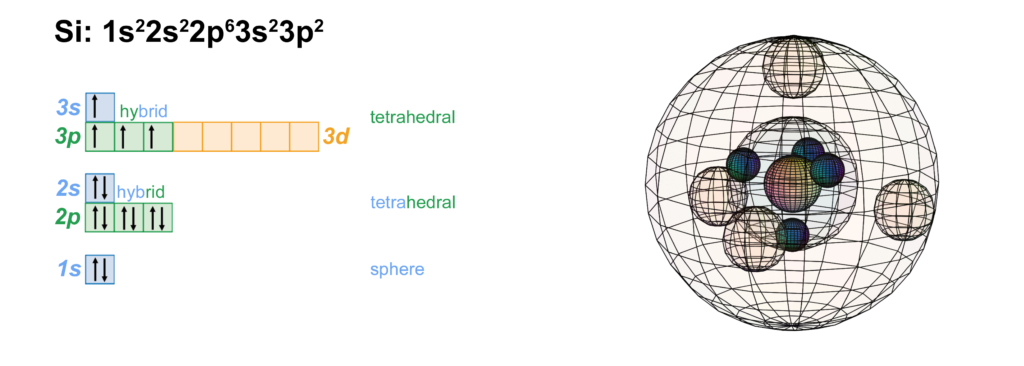
Silicon has the same electron structure as carbon, a hybridized, tetrahedral sp3 configuration. (The wireframe indicates the boundary of the n=3 shell.)
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell orbitals themselves will be more like spherical tetrahedra. The entire shell will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals, where electron density will be lowest (though not necessarily zero). Like argon, silicon features two nested spherical tetrahedra — an inner 2nd shell comprising 4 di-electrons, and an outer 3rd shell comprising 4 single electrons.

Bonding & Properties
Since silicon is a similar though larger atom than carbon, the four degenerate 3rd shell electrons form a less coherent quantum system than they do in carbon. The larger size gives pure silicon different (more semi-metallic) properties than carbon. This is because metals are characterized by the ease with which their electrons can delocalize from the atomic core. The less well-bound they are to the nucleus, the more metallic the nature of the element. This means elements become more metallic as we move down the periodic table (as their ionization energies decrease).
Like carbon silicon can make 4 covalent bonds, and its natural crystal form has the same network covalent structure as diamond. This gives it a high melting point, but not the same strength or hardness as diamond because, being larger, silicon does not bond with itself as strongly as carbon does. We also do not see many organic molecule analogues involving silicon in place of carbon.
Uses
With a lower electronegativity than carbon, oxygen bonds more strongly to silicon than to carbon, making silicates (sand) one of the primary components of earth’s crust. Due to its larger size and less coherent hybridization, along with the presence of a d-orbital in the 3rd shell into which to expand the electron octet, silicon is also capable of octahedral bonding. Silicon-based materials (like silicates) can therefore have more complex crystalline structures than carbon-based oxides.
Since silicon becomes less of an electrical resistor as temperature increases, it is a semiconductor, with important uses in electronics and photovoltaics.
RETURN to the Periodic Table
