
RETURN to Periodic Table
Cesium has one electron in the 6th shell. It has the same electron configuration as rubidium, but with five full shells within that have the identical configuration to xenon. Being one shell larger than rubidium, cesium has a lower ionization energy and is therefore even more reactive than the metals above it when forming the 1+ ion. Pure cesium metal reacts explosively with water and readily self-ignites in air as it reacts with (and donates its valence electron to) oxygen.
 CLICK HERE to interact with this object
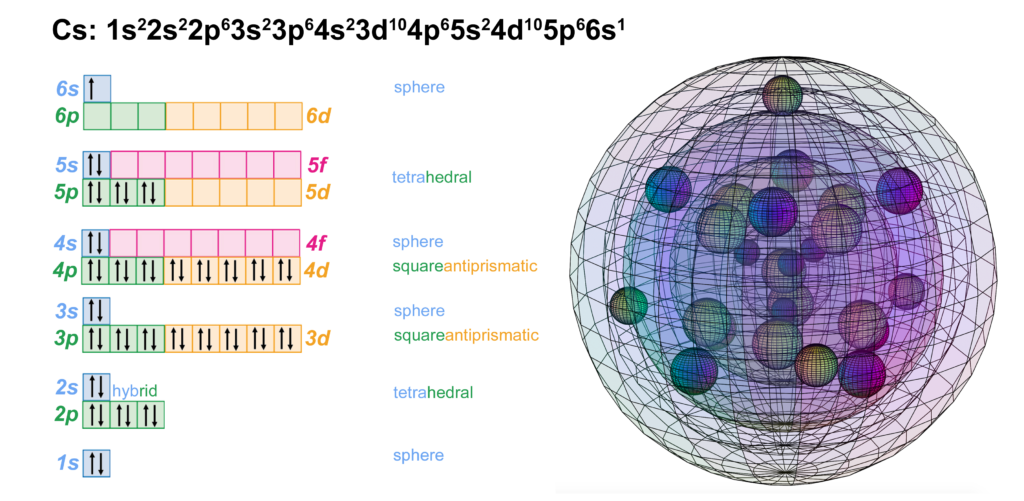
CLICK HERE to interact with this objectAn alternate view (shown below) of the orbitals shows an innermost 1st-shell sphere within a 2nd tetrahedral shell (dark blue) within a 3rd cubic anti-prismatic shell (light blue) within an anti-aligned 4th cubic anti-prismatic shell (brown) within a 5th tetrahedral shell (pink) within an unpaired 6s1 electron (light green).

Ion formation
With a lower ionization energy than rubidium, cesium will give up its valence electron even more eagerly than rubidium in an ionic interaction, in order to reach the stability of the 5s25p6 noble gas configuration of xenon, which is a multi-di-electron state with five concentric full shells. That is why cesium forms a 1+ ionic state so violently.
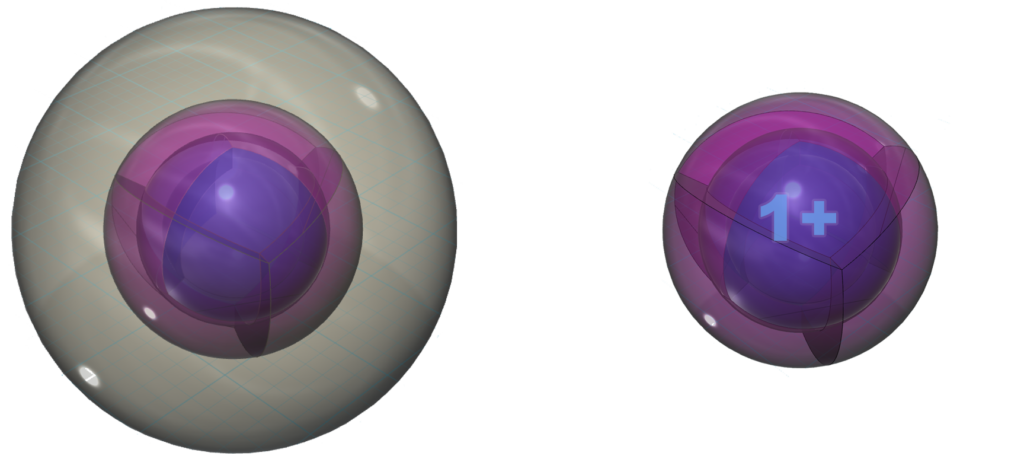
Pure cesium metal is very soft, melting at around room temperature (~250C). It is so reactive that it is considered pyrophoric, which means that it ignites spontaneously in air, and even in very cold (-1160C) water, as it donates its valence electron to oxygen.
A fun video showing cesium metal and its reactivity can be found HERE.
Uses
Cesium has several industrial and manufacturing applications, but perhaps its most interesting one is its use as the element in atomic clocks that keeps time.
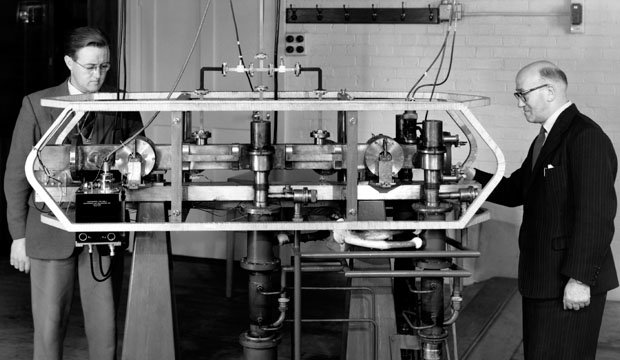
For an interesting video on how atomic clocks actually work, click HERE.
RETURN to the Periodic Table
OTHER GROUP I ELEMENTS: Lithium, Sodium, Potassium, Rubidium, Cesium
