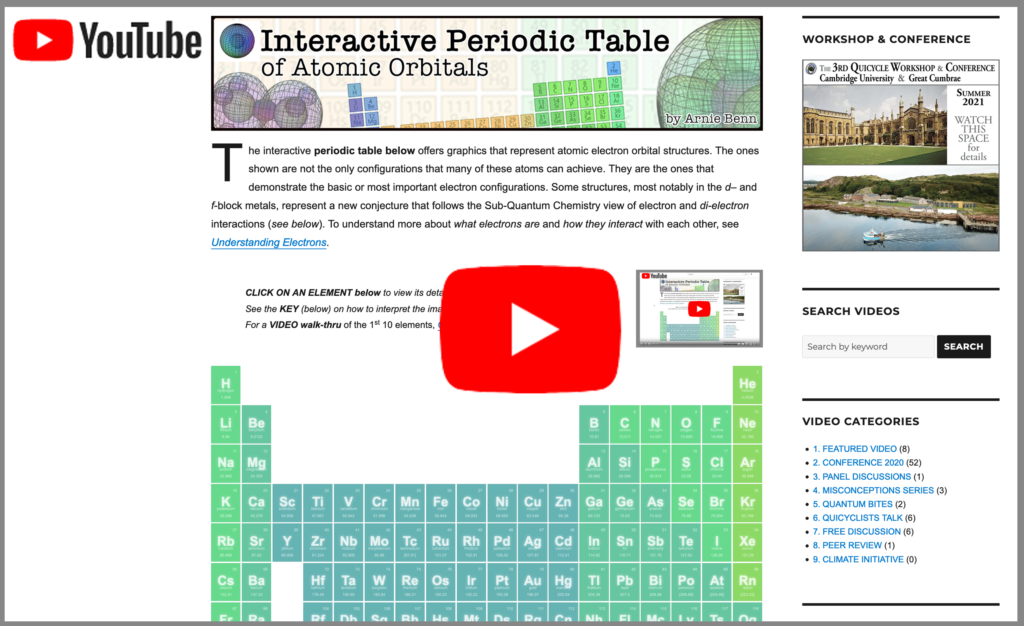
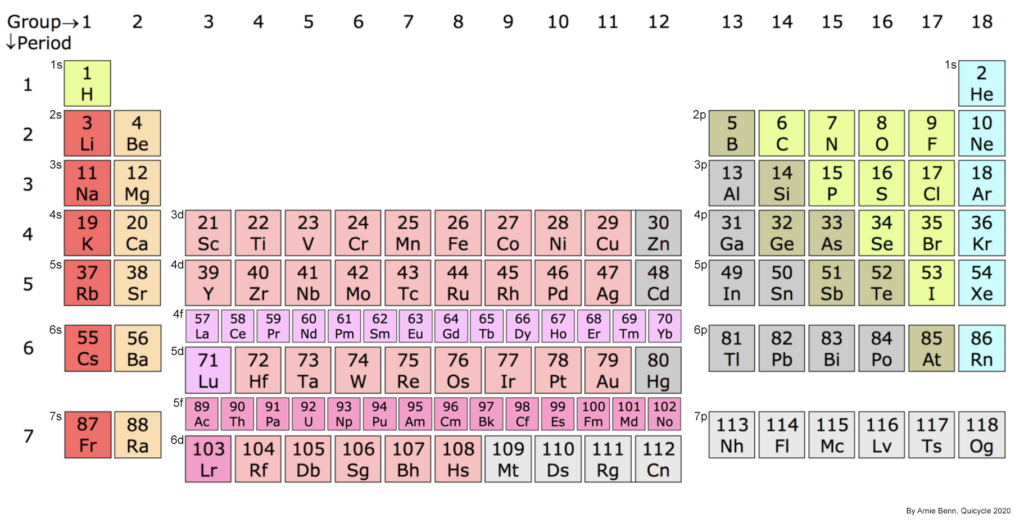
The interactive periodic table below offers graphics that represent the electron orbital structures in atoms. Some structures (such as those in the noble gases and the d– and f-block metals) are based upon a new conception that focuses on the electron and di-electron interactions implied by sub-quantum mechanics. (To understand more about what electrons actually are and how they interact with each other, see Understanding Electrons ![]() .)
.)
CLICK ON AN ELEMENT to view detail. (Only 1H – 71Lu so far.)
The KEY BELOW explains how to interpret the images & objects.![]() A VIDEO WALK-THRU of the 1st 10 elements is HERE
A VIDEO WALK-THRU of the 1st 10 elements is HERE ![]()
1.008
4.0026
6.94
9.0122
10.81
12.011
14.007
15.999
18.998
20.180
22.990
24.305
26.982
28.085
30.974
32.06
35.45
39.948
39.098
40.078
44.956
47.867
50.942
51.996
54.938
55.845
58.933
58.693
63.546
65.38
69.723
72.63
74.922
78.96
79.904
83.798
85.468
87.62
88.906
91.224
92.906
95.96
[97.91]
101.07
102.91
106.42
107.87
112.41
114.82
118.71
121.76
127.60
126.90
131.29
132.91
137.33
178.49
180.95
183.84
186.21
190.23
192.22
195.08
196.97
200.59
204.38
207.2
208.98
[208.98]
[209.99]
[222.02]
[223.02]
[226.03]
[265.12]
[268.13]
[271.13]
[270]
[277.15]
[276.15]
[281.16]
[280.16]
[285.17]
[284.18]
[289.19]
[288.19]
[293]
[294]
[294]
138.91
140.12
140.91
144.24
[144.91]
150.36
151.96
157.25
158.93
162.50
164.93
167.26
168.93
173.05
174.97
[227.03]
232.04
231.04
238.03
[237.05]
[244.06]
[243.06]
[247.07]
[247.07]
[251.08]
[252.08]
[257.10]
[258.10]
[259.10]
[262.11]
code source: @nemophrost
![]() Interactive Links: Orbital objects built using 3DCalcPlot
Interactive Links: Orbital objects built using 3DCalcPlot![]() can be rotated and zoomed.
can be rotated and zoomed.
KEY:
HYDROGEN (left): A lightly colored wireframe ![]() indicates a single electron in an orbital. In this case a sphere-shaped s-orbital.
indicates a single electron in an orbital. In this case a sphere-shaped s-orbital.
HELIUM (center): A full-color wireframe ![]() indicates a pair of electrons — a di-electron. The (phase) colors are not significant, just for ease of viewing.
indicates a pair of electrons — a di-electron. The (phase) colors are not significant, just for ease of viewing.
CARBON (right): An empty wireframe holds no electrons and shows just the outline of the (second) shell. (The inner full 1s2 shell looks the same as the helium di-electron.)
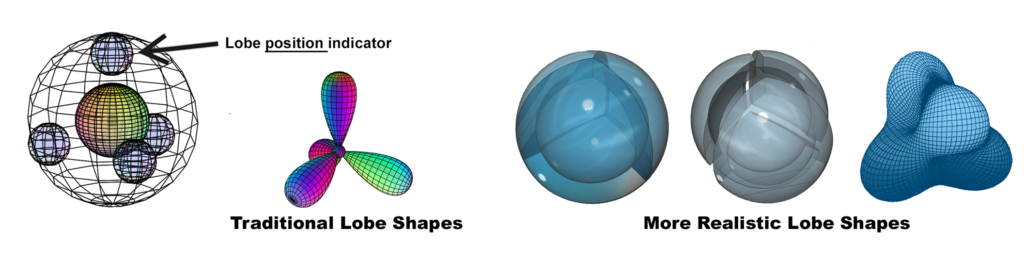
CARBON (left): Carbon ![]() has 4 unpaired 2nd shell electrons arranged in tetrahedral sp3 symmetry. The small outer spheres represent directions for the orbitals, not their shape. Only s-orbitals are actually spherical. A more realistic approach to the orbital shapes is shown on the right above.
has 4 unpaired 2nd shell electrons arranged in tetrahedral sp3 symmetry. The small outer spheres represent directions for the orbitals, not their shape. Only s-orbitals are actually spherical. A more realistic approach to the orbital shapes is shown on the right above.
CHEMISTRY DEFINITIONS:
Definitions of some basic chemistry terms can be found in the Glossary. ![]()
SOME MOLECULES & SUBSTANCES:
Dihydrogen (H2)
Water (H2O)
Sodium chloride (NaCl) — salt
Ammonia (NH3)
Methane (CH4)
Dioxygen (O2)
Ozone (O3)
PERIODIC TRENDS:
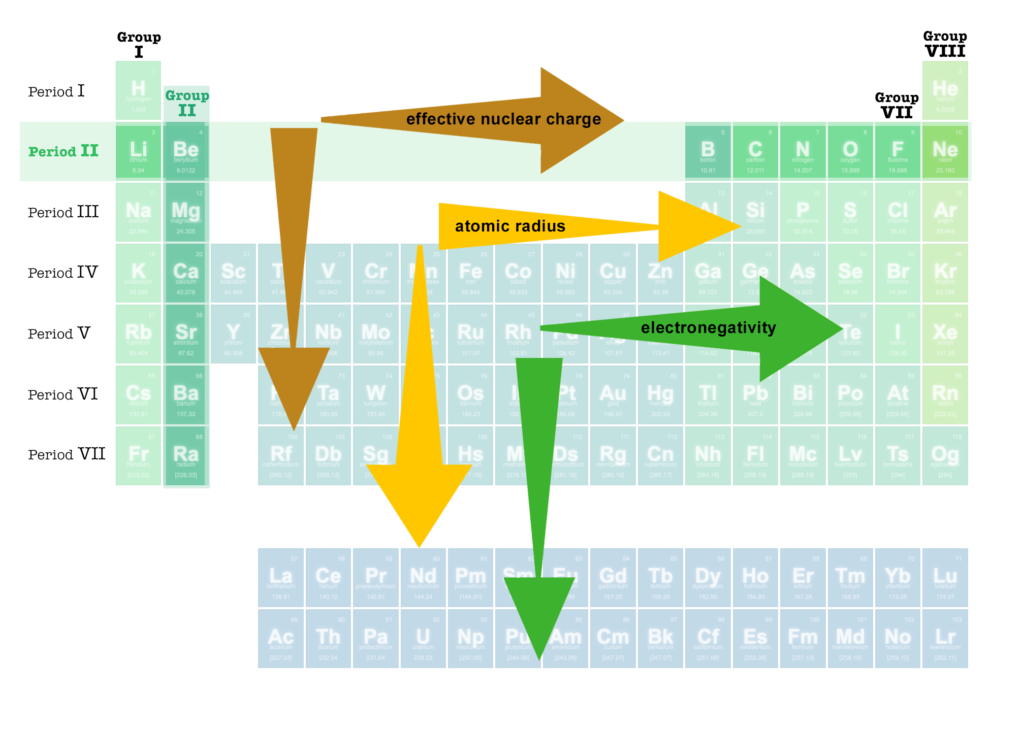
Certain atomic properties vary in a roughly consistent way when we move across a PERIOD (row) or down a GROUP (column). These include effective nuclear charge, atomic radius, ionization energy, and electronegativity:
– PERIOD II ![]()
– PERIOD III ![]()
– GROUP I
– GROUP II
– GROUP VII
– GROUP VIII
PLATONIC GEOMETRY:
Atomic orbitals must achieve spherical symmetry, and platonic geometry forms the foundation of such symmetry.
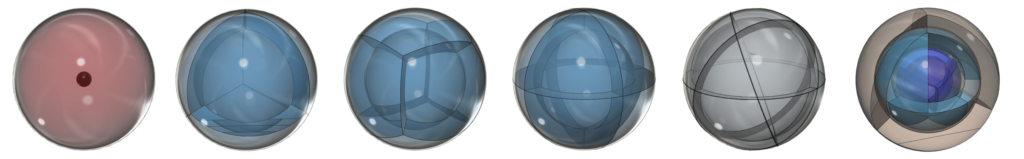
![]() Click here to see an innovative lecture series on spherical and platonic geometry by Gary Doskas
Click here to see an innovative lecture series on spherical and platonic geometry by Gary Doskas ![]()
![]() (see Article (2011)
(see Article (2011)

Some of the structures in this periodic table, most notably from the d– and f-block metals, represent a new conjecture based upon recent advances in sub-quantum mechanics. The primary difference between this approach and the traditional approach centers around the electron and di-electron interactions that take place between electrons in different sub-shells within the same shell, as well as between electrons in adjacent shells. (For more detail, see Understanding Electrons).
This approach suggests that the quantum interactions between electrons in close proximity play a central role in determining both atomic and molecular geometry. These quantum interactions include spin-bonding, spin exclusion, field cancellation, and orbital hybridization. They yield symmetrical, phase-locked, resonant, coherent, spherically-harmonic, stationary electron waves that represent the lowest energy state of the system.
These symmetrical wave resonance structures appear to account for certain magnetic trends across the transition metals and rare earth metals, specifically that of magnetic (susceptibility) strength (shown below for the d-block). By way of example, why does scandium (Sc) have a higher paramagnetic strength with 1 unpaired electron than vanadium (V) has with 3 unpaired electrons, and why does palladium (Pd) (with supposedly no unpaired electrons) have a higher value than manganese (Mn) with 5 unpaired electrons? (See more detail HERE.)
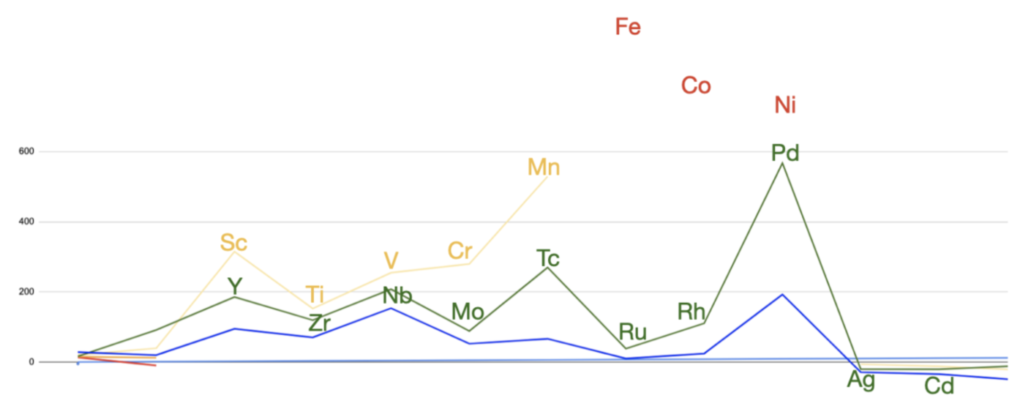
This approach seeks to clarify the mechanism leading to paramagnetism versus that leading to ferromagnetism, based upon the electron geometry and quantum interactions occurring within atomic orbitals. In the process, we also seek to account for the nature of the dicarbon (C2) molecule’s observed ‘quadruple bond,’ as well as the strong paramagnetism of the oxygen molecule (O2).
We hope you find this interactive periodic table a useful resource. Best wishes. Arnie Benn, Quicycle, CA
BACK to the top