
RETURN to Periodic Table
Phosphorus is the 15th element on the periodic table. It has 15 protons and 16 neutrons in the nucleus, giving it a mass of 31 amu, and it has 15 electrons enveloping the nucleus.
Electron Shell
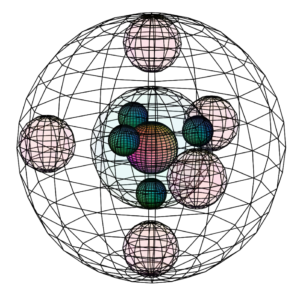
Phosphorus has the same tetrahedral sp3 electron configuration as nitrogen, though, as a larger atom, the chemistry of phosphorus has important differences from that of nitrogen. With an available d-orbital into which to hybridize, phosphorus can achieve a greater variety of molecular geometries than can nitrogen. (The wireframe indicates the boundary of the n=3 shell.)
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell orbitals themselves will be more like spherical tetrahedra. The entire shell will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals, where electron density will be lowest (though not zero). Like argon, phosphorus features two nested spherical tetrahedra — an inner 2nd shell comprising 4 di-electrons, and the outer 3rd shell comprising 1 di-electron and 3 single electrons. In this case the orbital containing the di-electron (dark pink) will occupy slightly more volume than the three containing an unpaired electron.
Bonding & ion formation
Phosphorus is keen to obtain three extra electrons to fill its 3rd shell, though it is not as pressing of an issue for phosphorus as it is for nitrogen since the nuclear charge is lower at the boundary of the larger atom. Phosphorus can make one or more covalent bonds or it can gain three electrons in ionic interaction in order to reach the stability of the 3s23p6 noble gas configuration of argon, which is a multi-di-electron state with three concentric full shells. That is why phosphorus forms a 3– ionic state. The negative ion is much larger than its neutral version because electrons now outnumber protons by three. This results in a much lower effective nuclear charge — a lower average attraction by the nucleus on each electron, which expands the size of the electron shell as it is attracted inward with less force.

But if phosphorus is approached by highly electronegative atoms like oxygen or chlorine, they can induce phosphorus’s paired valence electrons to uncouple, yielding 5 unpaired electrons available for bonding. This is only possible because the 3rd shell contains a d-orbital. While phosphorus does not usually have electrons in its 3d-orbital, the 3rd shell has enough volume to accommodate those orbitals. This allows phosphorus to hybridize into that space and to make up to 5 covalent bonds, as we see in the trigonal bipyramidal PCl5 molecule.

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectUses
Since phosphorus has a lower electronegativity than nitrogen, it’s outer electrons are not as well bound. Oxygen will therefore react more easily and strongly with phosphorus than with nitrogen, which is why phosphorus burns (combusts) so readily. It can therefore be used to make matches and incendiary devices.
The most stable form of phosphorus in nature is the phosphate ion (PO43–). When three electrons are made available by nearby metal atoms, 1 phosphorus atom and 4 oxygen atoms can form a symmetrical, tetrahedral arrangement where every atom has a full and symmetrical outer electron shell. Phosphates are a key nutrient in nature. They are commonly found in rocks, from where they are released by erosion, and they also help make up the ‘backbone’ of the DNA molecule in all living things.
RETURN to the Periodic Table
