
RETURN to Periodic Table
Helium is the 2nd element on the periodic table because it has 2 protons in its nucleus. Since the protons carry a positive charge, they will attract 2 electrons in order to balance the charge. Helium is therefore a neutral atom overall, with two electrons surrounding two protons (and 2 neutrons) in the nucleus.
Electrons are ignored when considering the mass of the atom because they are over 1,800 times lighter than protons and neutrons. With 2 protons and 2 neutrons, helium has an atomic mass of 4 amu.
Electron Shell 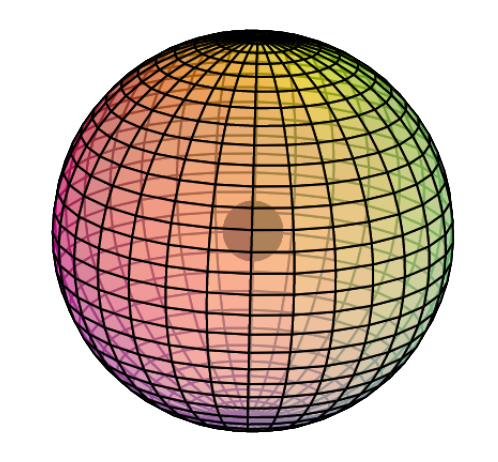
Helium only has 1 electron shell, a spherical s-orbital, and it contains 2 electrons — a di-electron. We describe it with an electron configuration of 1s2. The “1–s–2” means: shell number 1, the s-orbital, which contains 2 electrons. The full-color wireframe (below) represents a di-electron.

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: Even though it is often useful to talk about electron orbitals (and even subatomic particles for that matter) as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in a single harmonic wave structure.
A particle is a perfectly-struck electromagnetic ‘note’; an atom is a perfectly-struck electromagnetic harmony between electromagnetic ‘knots’.
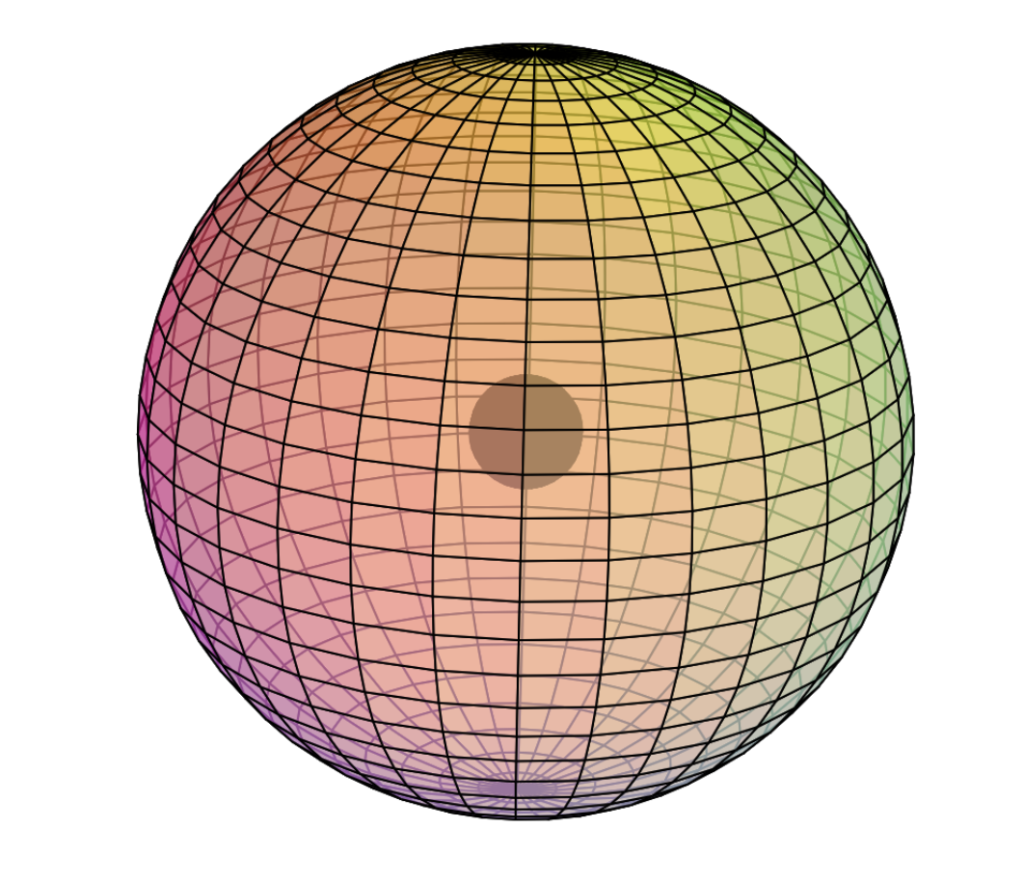
The image below provides a different view of the spherical helium atom. Note that the size of the nucleus in the center is greatly exaggerated. If the electron cloud were the size of a large football stadium, the nucleus would be the size of a penny at the center of the field — barely visible.
As in the case of hydrogen (H), an isolated proton is about 100,000 times smaller than the scale of an atom’s electron cloud. (As we saw, though, a quantum mechanical view might paint a slightly different picture of the sizes of the proton and electron when in a mixed, atomic state.)


Helium has the smallest atomic radius on the periodic table. (This square represents the size of the largest atom, Francium, Fr).
Helium is completely non-metallic. It has no electronegativity value, given that it has a full electron shell and will therefore neither gain nor lose electrons. It will therefore not bond (react) with any other atom.
Helium is the smallest atom — smaller than hydrogen — because it has twice as many protons in the nucleus attracting twice as many electrons inwards. This higher “effective nuclear charge” shrinks the atom’s size. Since helium’s electrons are the most closely bound to their nucleus, helium is also the element with the highest ionization energy (2,370 kJ/mol or 24.5 eV) and is consequently the most unreactive element on the periodic table.
Similar to the di-electron that envelops and binds the hydrogen molecule (H2), helium’s two electrons form a very stable and perfectly spherical di-electron state — a boson state — where the two electron wave functions are completely superimposed upon one another in a magnetically anti-parallel fashion. (See Understanding Electrons.)
Helium is called a ‘noble’ gas because of its non-interaction with other atoms. This causes it to be a gas under all but the most extraordinary conditions (of low temperature and high pressure).
Nucleus
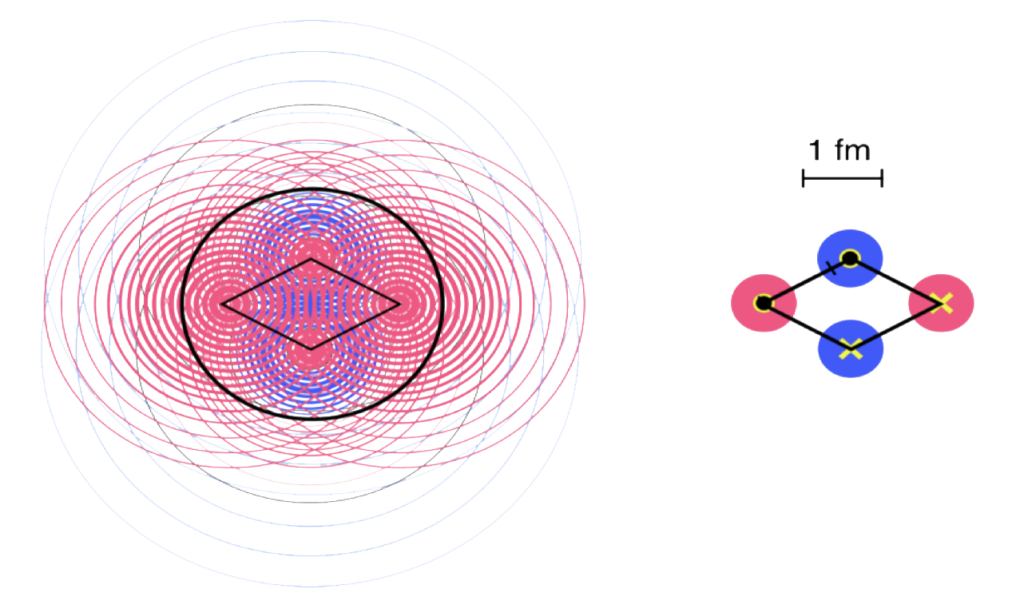
Helium also contains the most stable nucleus — the alpha particle. Most other nuclei are composed of various combinations of alpha particles (see the Robinson Model of Nuclear Binding), which is why this is the only type of multi-nucleon structure that is ejected during radioactive decay.
![]() DELVING DEEPER:
DELVING DEEPER:
As a quantum system, the helium atom is significantly different to the hydrogen atom.
It is a smaller atom than hydrogen as a result of its stronger nuclear charge drawing its electron charge density inward more strongly.
It is also an overall spin-zero state. With two proton-neutron pairs, and with one of each spin for each, the helium nucleus is a di-boson. (See diagram above.) Adding a di-electron shell makes the helium atom an exceptionally stable tri-boson state.
Uses
Hydrogen (H2) and helium (He) are the two lightest gases. Since their atoms have the lowest masses, their gases have the lowest densities. This makes them the best gases to use for buoyancy — in balloons and blimps — in the atmosphere. Since hydrogen gas is so highly flammable, however, safety requires that helium be the gas of choice for buoyancy. Helium will never ignite because, due to its di-electron stability, it is not interested in reacting with oxygen (or any other atom).
RETURN to the Periodic Table
SEE OTHER NOBLE GASES: Helium, Neon, Argon, Krypton, Xenon
