
RETURN to Periodic Table
Beryllium is the 4th element on the periodic table because it has 4 protons in its nucleus. The positive protons will attract 4 electrons to surround the nucleus in order to form a neutral atom. With 4 protons and 5 neutrons, most beryllium atoms have an atomic mass of 9 amu.
Electron Shell
Beryllium has 4 electrons. The first two occupy an inner core shell, which is the 1s2 di-electron, just like helium’s. This first shell is surrounded by two more electrons in a 2nd shell s-orbital, forming a 2s2 di-electron. This makes beryllium less willing to lose an electron than lithium, giving beryllium a higher ionization energy and making it less reactive than lithium.
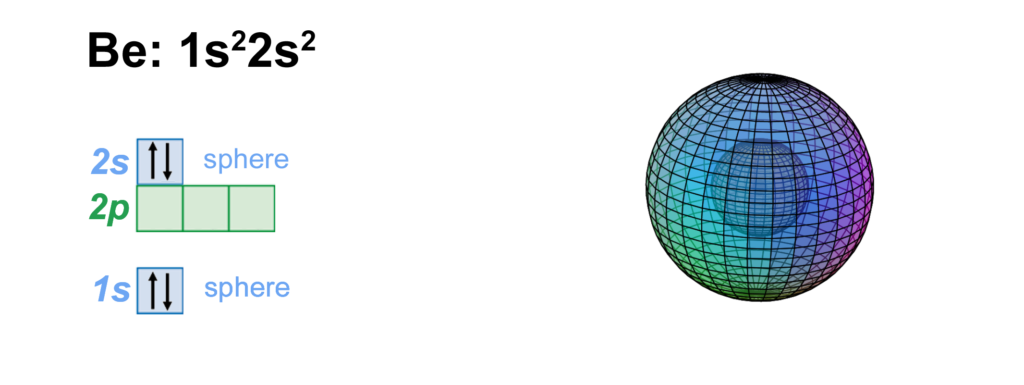
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.This image provides another view of the spherical beryllium atom. The 2nd shell introduces a significantly larger volume to the atom, which is why it can accommodate four times as many electrons as the 1st shell.

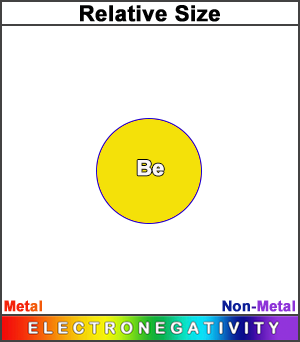
Beryllium has a smaller atomic radius than lithium. (This square represents the size of the largest atom). The decrease in size, as we move across the second row of the periodic table, results from an increase in effective nuclear charge.
Its electronegativity value makes it a metal, though slightly less metallic than lithium. This is because its stronger effective nuclear charge, not to mention the stability of its 2nd shell di-electron, makes it hold its electrons more strongly.
Ion formation
While beryllium can become an ion, it does so less readily than magnesium (which has the same electron configuration but is one shell larger). Beryllium can be convinced to lose its two valence electrons to more electronegative atoms in an ionic interaction, and it will lose both at the same time in order to reach the stability of the 1s2 di-electron state, like helium. That is why beryllium forms a 2+ ion. The positive ion is much smaller than its neutral version because protons now outnumber electrons by two. This results in a much higher effective nuclear charge — a higher average attraction by the nucleus on each electron, which shrinks the size of the electron shell as it is attracted inward with more force.
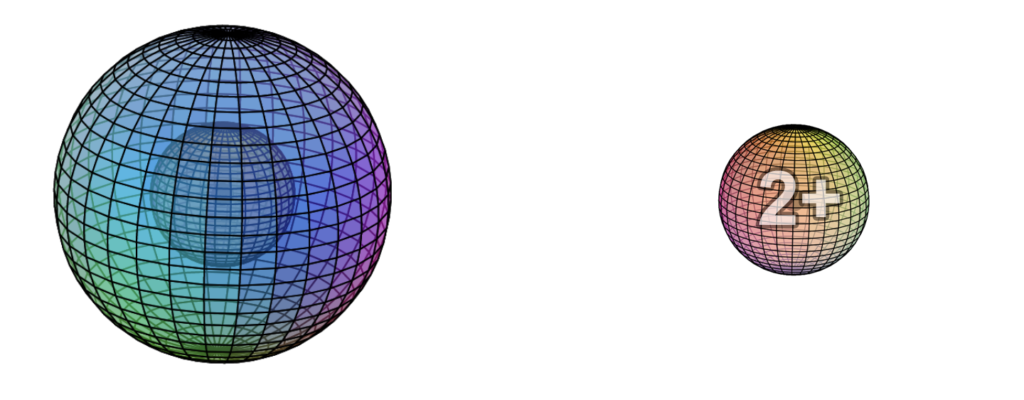
Magnetic properties
Beryllium is also the only metal in Group II that is diamagnetic — meaning that it repels away from any type of magnetic field. This is usually due to the presence of di-electrons in the electron shell, which cause the atom to repel from a magnetic field in order to maintain their perfect field cancellation.
Metals, however, are usually paramagnetic. For beryllium to be diamagnetic, we might conjecture that its valence electrons are somehow retaining some of their di-electron character, despite being (‘delocalized’) in the metallic (crystal) state. Delocalization must be present because beryllium conducts electricity. The fact that it forms a hard but brittle metal at room temperature might imply that less than all of its valence electron density is participating in the metallic bonding matrix. This may also account for beryllium exhibiting superconductive properties at very low temperatures. (Compare: aluminium (Al) magnetic properties.)
RETURN to the Periodic Table
OTHER GROUP II ELEMENTS: (Helium), Beryllium, Magnesium, Calcium, Strontium, Barium
