
RETURN to Periodic Table
Lithium is the 3rd element on the periodic table because it has 3 protons in its nucleus. Since the protons carry a positive charge, they will attract 3 electrons to surround the nucleus in order to balance the charge and form the neutral lithium atom. With 3 protons and 4 neutrons, most lithium atoms have an atomic mass of 7 amu.
Electron Shell
Lithium has three electrons. The first two occupy an inner core shell, which is the 1s2 di-electron, just like helium’s. This first shell is surrounded by a single electron in a 2nd shell s-orbital. This unpaired 2nd shell electron makes lithium somewhat reactive since it would rather all its electrons were paired. It will therefore seek to lose this unpaired 2s1 electron in an ionic interaction (see below).
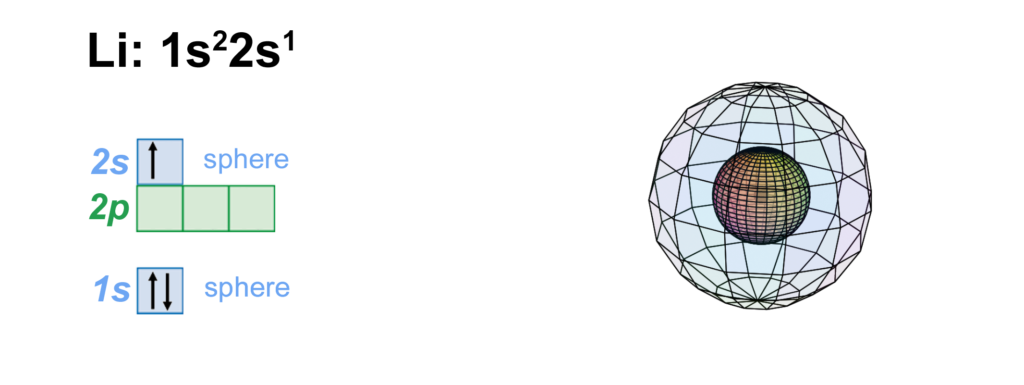
 CLICK HERE to interact with this object.
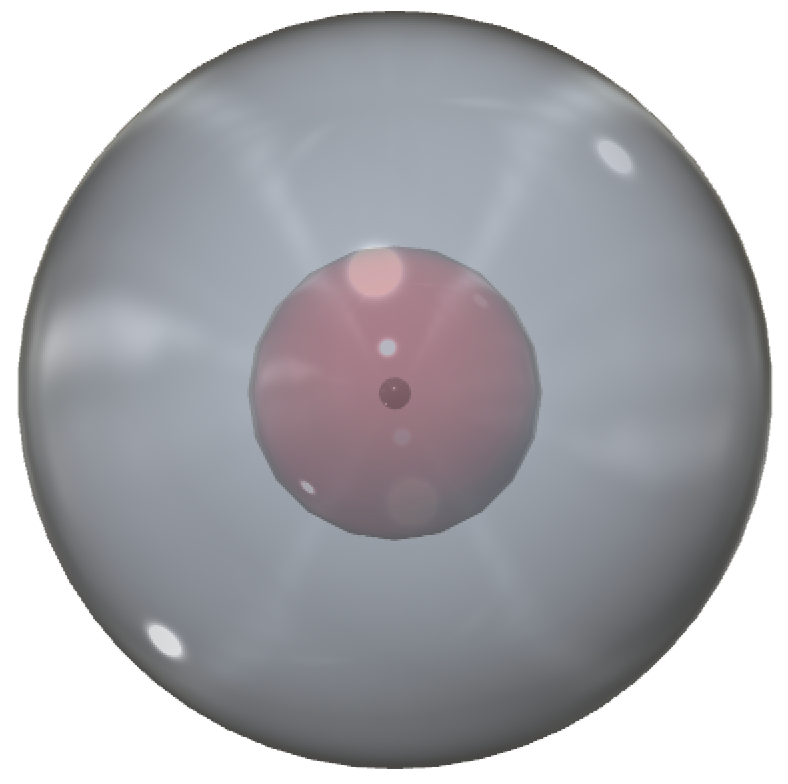
CLICK HERE to interact with this object.This image provides another view of the spherical lithium atom. The 2nd shell introduces a significantly larger volume to the atom, which is why it can accommodate four times as many electrons as the 1st shell.
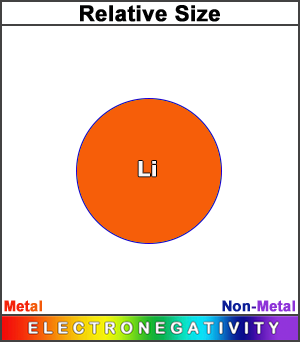
Lithium has a significantly larger atomic radius than hydrogen and helium. (This square represents the size of the largest atom — Francium, Fr).
Its electronegativity value makes it a metal, and with a much more metallic character than hydrogen, given that lithium, with an extra shell, gives an electron away more readily.
Ion Formation
When lithium loses its valence electron in an ionic interaction, it achieves the stability of the 1s2 di-electron state, the highly stable electron state we find in helium. That is why lithium forms a 1+ ion. The positive ion it becomes is smaller than the neutral atom because protons now outnumber electrons by one. This results in a higher effective nuclear charge — a higher average attraction by the nucleus on each electron, which shrinks the size of the electron shell as it is attracted inward with more force.

In water, pure lithium reacts mildly as it forms the 1+ ion and dissolves. Since lithium has a lower electronegativity that hydrogen, it is an easier source of electrons for the highly electronegative oxygen atom in the water. Each water molecule (H2O) therefore ejects a hydrogen atom in favor of a lithium atom, forming the alkaline lithium hydroxide solution. Bubbles of hydrogen gas (H2) are released from the water in the process:
2Li(s) + 2H2O(l) —> 2LiOH(aq) + H2(g)
Uses
One of the most common uses for lithium is in the manufacture of batteries. Because lithium is so willing to lose its outer (valence) electron, it has the lowest standard reduction potential (E0) with a voltage of -3.04V.
Lithium therefore makes for a very good source of electrons when paired with another atom or molecule that is seeking to gain electrons. This creates a flow of electrons — electricity.

(Source: Turbotom1 at English Wikipedia, CC BY-SA 3.0)
RETURN to the Periodic Table
OTHER GROUP I ELEMENTS: Hydrogen, Lithium, Sodium, Potassium, Rubidium, Cesium
