
RETURN to Periodic Table
Barium has an s-orbital di-electron in the 6th shell. It has the same electron configuration as strontium, but with five full shells within that have the identical configuration to xenon. Being one shell larger than strontium, barium has a slightly lower ionization energy and is therefore slightly more reactive and more soluble than calcium and strontium, allowing it to form the 2+ ion more readily.

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectBarium’s outermost di-electron forms the 6th shell, finding these valence electrons further from (and more shielded from) the charge of the nucleus. We would therefore expect this di-electron to be less well-bound than the 5th or 4th shell di-electrons that we find in the outermost shells of strontium and calcium. The reason is that the 6th shell’s electron density fills a larger orbital volume. This should cause barium to be more paramagnetic — with a higher magnetic susceptibility (χm) value — than strontium or calcium above it on the periodic table. If electrons are further from the nucleus and less well bound, they will be more responsive to an external magnetic field. The other χm values on the periodic table reinforce this trend. But surprisingly, barium is less paramagnetic (with χm=+20) than both strontium (χm=+92) or calcium (χm=+40) above it.
This should either mean that the outer 6s2 di-electron is more well-bound than the di-electrons of the 5s2 and 4s2 orbitals, which seems unlikely, or that something else about the electron resonance of barium gives it added stability and coherence. This might arise from barium’s unique and highly symmetrical electron configuration, depicted below.
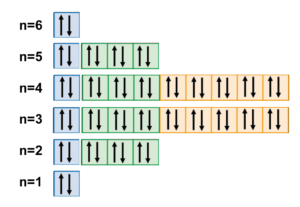
Interestingly, this symmetry seems to create a banded shell structure in the electron cloud. It has a virtual shell of highest electron density made up of the full 3rd and 4th shells, with decreasing electron density as we approach either the nucleus or the outer “boundary”.
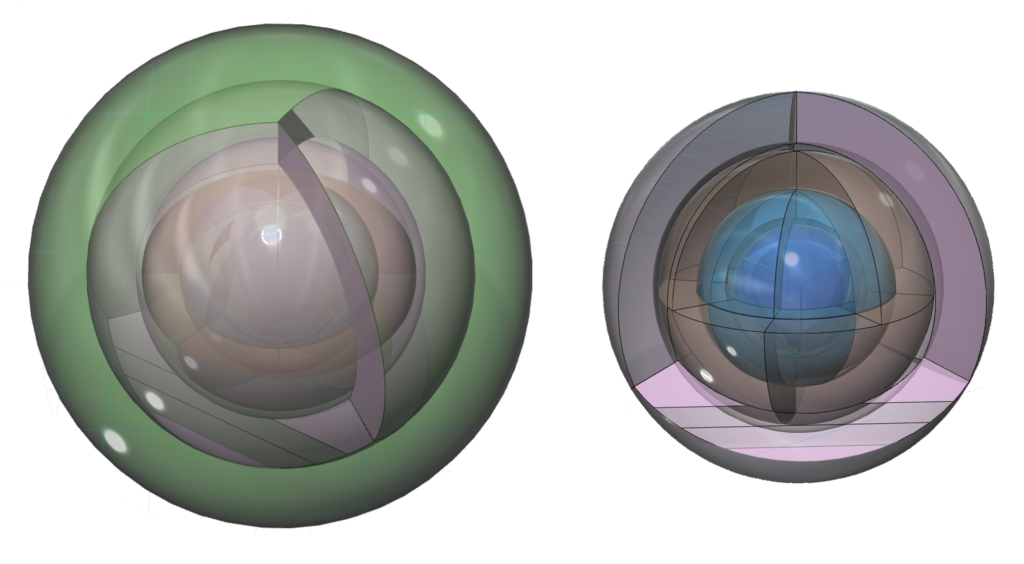
An alternate view (shown below) of the orbitals shows an innermost 1st-shell sphere within a 2nd tetrahedral shell (dark blue) within a 3rd cubic shell (light blue) within a 4th cubic shell (brown) within a 5th tetrahedral shell (pink) within a 6s2 spherical di-electron (dark green).
RETURN to the Periodic Table
OTHER GROUP II ELEMENTS: Beryllium, Magnesium, Calcium, Strontium, Barium
