
RETURN to Periodic Table
Sodium is the 11th element on the periodic table. It has 11 protons and 12 neutrons in the nucleus, giving it a mass of 23 amu, and it has 11 electrons enveloping the nucleus.
Electron Shell
Sodium has a single valence electron in its 3rd shell with two full core shells within that have the identical configuration to neon. This makes sodium keen to donate its single valence electron in order to regain the electron symmetry of neon, resulting in its 1+ ionic character when interacting with other non-metal atoms. Being larger than lithium or hydrogen, the lower electrostatic force from the nucleus and the greater core electron shielding cause sodium’s valence electron to be more weakly bound, and this makes sodium more reactive than lithium. Pure sodium metal reacts violently, sometimes explosively, when placed in water as it donates its valence electron to oxygen. The heat of this reaction ignites the hydrogen gas that is also produced, burning with a yellow flame.
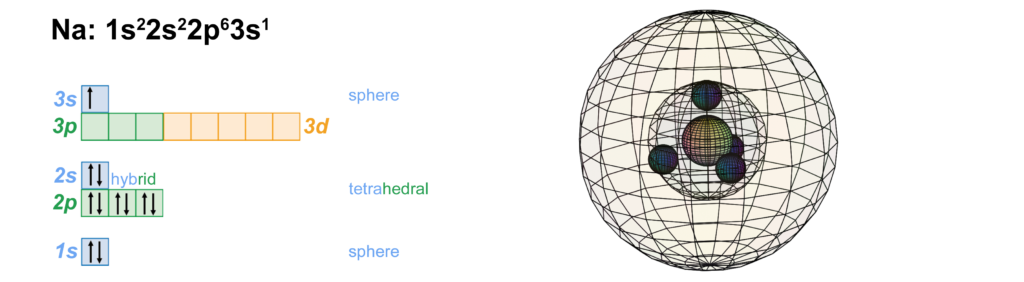
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.As we saw in the case of neon, the 2nd shell orbitals are more like spherical tetrahedra, and the 3rd shell is a single electron in a spherical s-orbital. These orbitals represent phase-locked, resonant, coherent, harmonic, stationary waves.
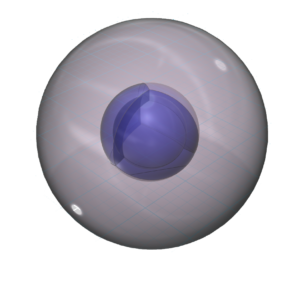

Sodium has a larger atomic radius than lithium. (This square represents the size of the largest atom).
Its lower electronegativity value results from its larger size and low effective nuclear charge, making it such a reactive metal.
Ion Formation
Sodium will give up its valence electron readily in an ionic interaction in order to reach the stability of a full 2nd shell. This is the same electron configuration as the 2s22p6 noble gas configuration of neon — a multi-di-electron state with two concentric full shells. That is why sodium forms a 1+ ionic state.
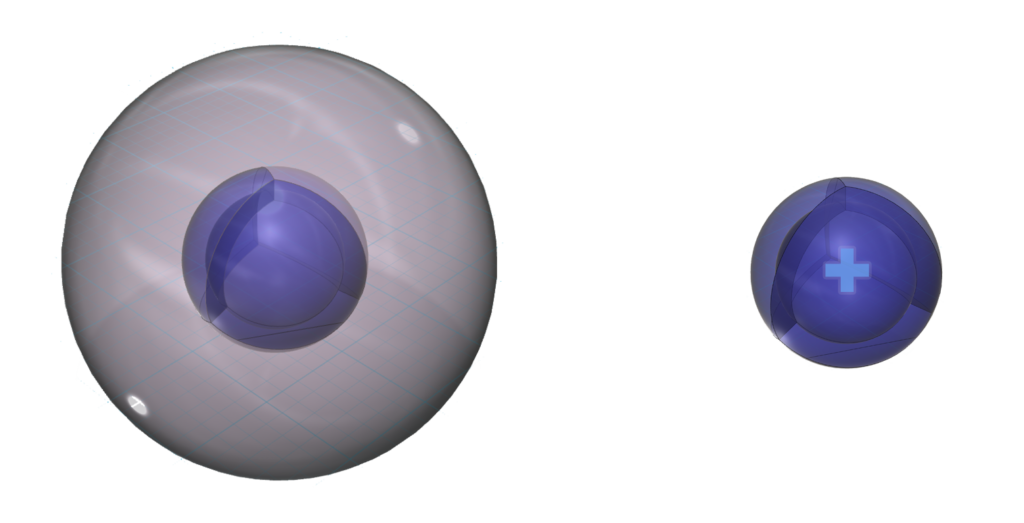
Salt
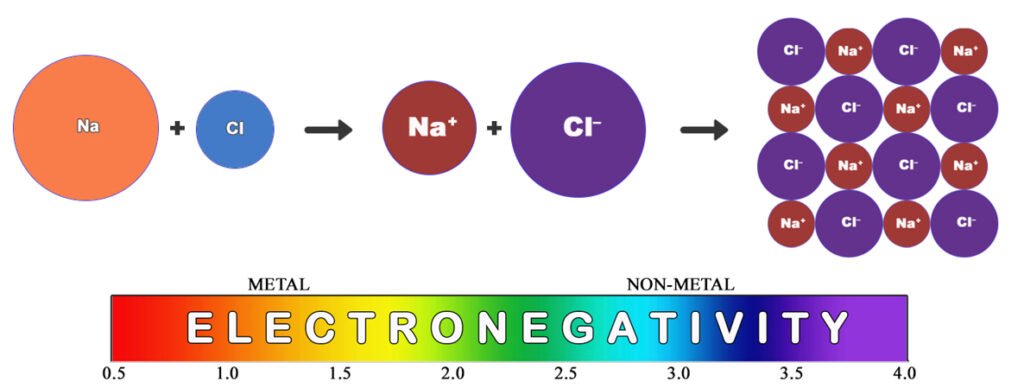
When sodium and chlorine interact, sodium gives the electron it wants to lose to chlorine, which is keen to gain it. This forms both atoms into their ions and allows both to achieve full shell configurations. The ions can then stick to each other because of their opposite charges, forming sodium chloride (NaCl) crystals. This process is called ionic bonding, and it occurs between a metal (from the left side of the periodic table) and a non-metal (from the right side). The term “salt” can also be used to apply to any ionic crystal.
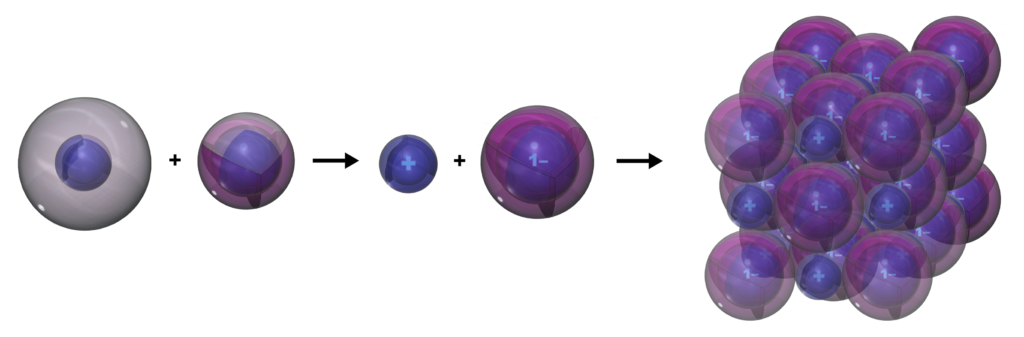
Below is another visualization of the formation of sodium chloride from the point of view of electronegativity. The electrons in a bond will always be pulled more strongly towards the atom that is closer to the violet end of the electronegativity spectrum. In this case, there is such a great difference between how strongly they hold their electrons that the chlorine will be able to strip the sodium’s electron completely away. This clarifies why the two ions form, as opposed to them still sharing the electrons (as occurs in water).
Sodium chloride (NaCl) dissolves in water because the polar H2O molecules and the ions in the crystal attract each other. The water molecules can therefore tug ions off the crystal and still satisfy the ion’s desire to attract their opposite polarity. As each ion leaves the crystal, it becomes hydrated — surrounded by water molecules.
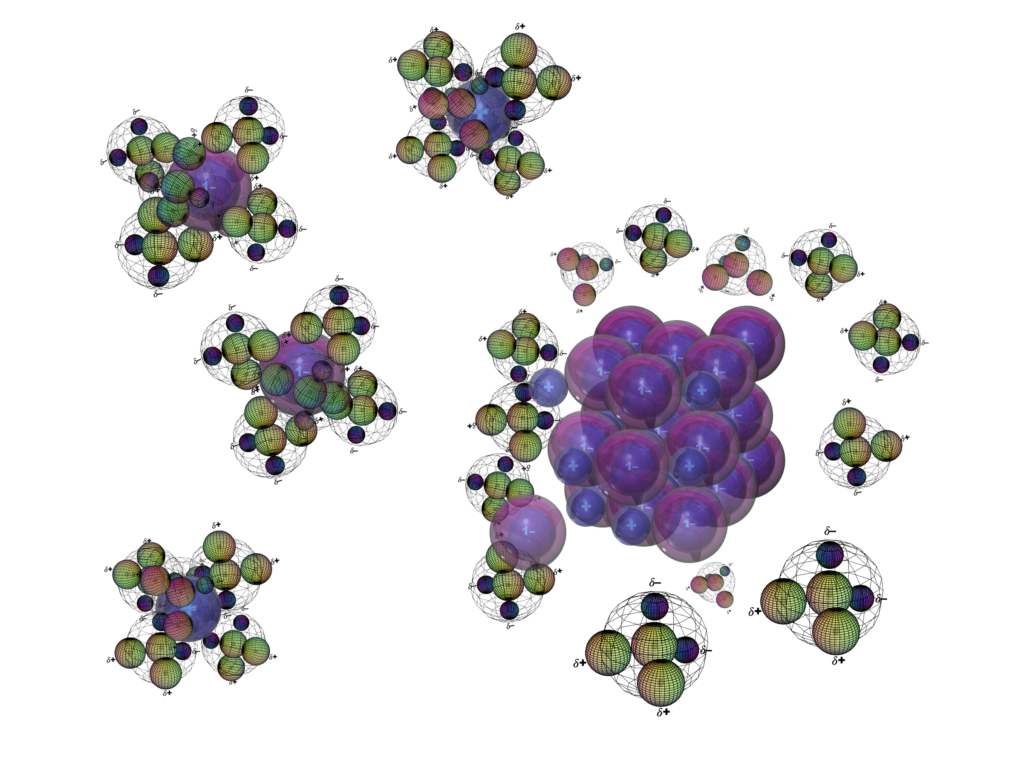
When the water is allowed to evaporate from the salt solution, the ions become increasingly exposed to one another, and the solid crystals re-form due to electrostatic attraction.
Although the ocean contains many different ions and just about every element on the periodic table (in trace amounts), it is mainly made up of water (H2O) and sodium chloride (NaCl). Of these four elements, the mass of sea water is made up of about 86% oxygen, 11% hydrogen, 1.9% chloride, and 1.1% sodium.
RETURN to the Periodic Table
OTHER GROUP I ELEMENTS: Lithium, Sodium, Potassium, Rubidium, Cesium
