
RETURN to Periodic Table
Below: Electron Shell, Bonding & Ions, Magnetic Properties, Temperature, Other Magnetism
Iron is the 26st element on the periodic table. It has 26 protons and 30 neutrons for a mass of 56 amu, and 26 electrons.
Electron Shell 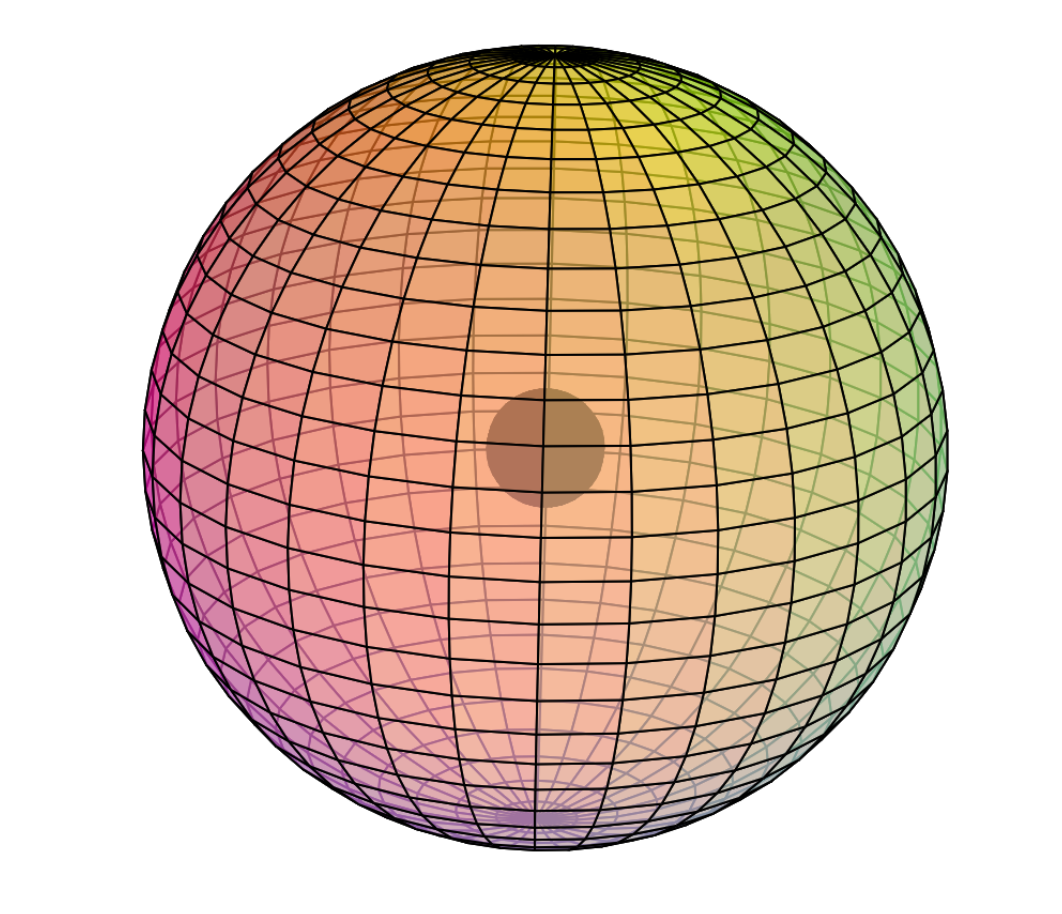
DISCLAIMER: The following reflects the sub-quantum mechanics approach to electron interactions and hybridization. Some details may therefore differ somewhat from traditional quantum chemistry:
Iron is the sixth element with electrons in the d–orbital. Building upon the pd-hybridization [ref] we introduced in regard to the previous d-block transition metals, it is proposed that iron has a 3rd shell containing 4 di-electrons and 4 unpaired electrons in p3d5-hybridized, cubic symmetry resonating within a spherical 3s2 orbital. The 4 di-electrons and 4 unpaired electrons will arrange themselves in a highly symmetrical, alternating fashion that minimizes repulsion. This structure (shown below) can also be viewed as two intersecting, antiparallel (antiprismatic) tetrahedra, one containing di-electrons, the other single electrons.
In such a configuration, the 4 tetrahedral 2nd shell di-electrons will align themselves directly beneath the single electrons in the 3rd shell in order to minimize di-electron repulsion between shells.

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectNOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell hybrid orbitals themselves will form a cubic arrangement that divides the (cuboctahedral) shell into eight equal volumes, with two 4-way symmetries. The entire shell will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional hybrid orbital lobe shapes) and will decrease toward the nodal regions between orbitals — as wave structures usually do — where electron density will be lowest (though not zero).
NOTE ALSO: Even though it is often useful to talk about these orbitals as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in this harmonic wave structure.
The diagrams below show iron’s eight 3rd shell p3d5-hybrid orbitals. (The darker blue color represents di-electron orbitals, the lighter blue color represents unpaired electron orbitals.) In reality, the eight volumes will not be exactly equal in size because di-electron orbitals (with charge 2-) will be larger and will repel the unpaired electron orbitals (with charge 1-) more strongly, constricting them. It is therefore proposed that iron’s unpaired core electrons will form a perfect tetrahedral geometry with respect to one another, due to the (tetrahedral) symmetry of the constriction around (and between) them, and that they will become extended, radially outward, as a result of the repulsion from both beside and beneath them.
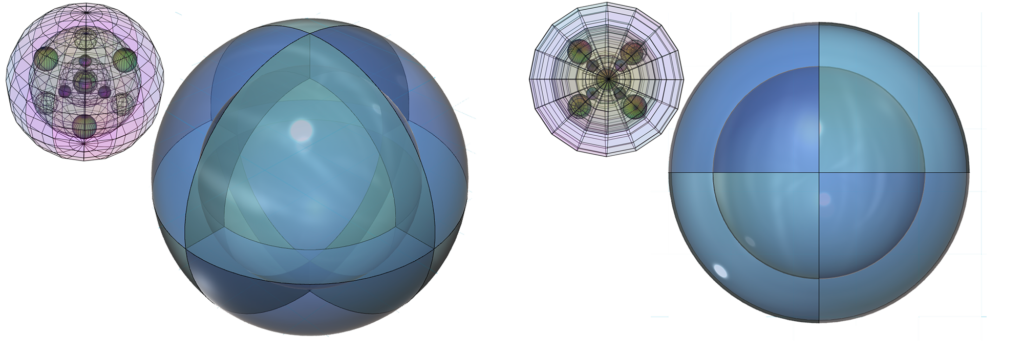
This double antiparallel tetrahedral symmetry of iron’s 3rd shell is also known as a dual tetrahedron, in which each point also coincides with a corner of a cubic structure. Two different views of this are offered below.
Bonding & Ion Formation 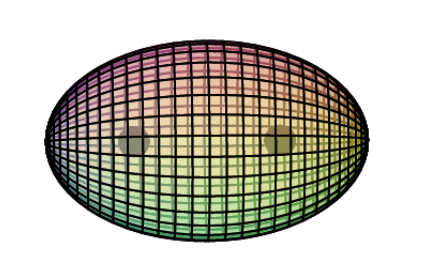
When iron atoms bonds with other metal atoms in a solid, they form a crystal structure in which their valence electrons become ‘delocalized’ — shared into a matrix of electron density within which the now-positive atomic cores remain suspended. They are held in their relative positions by a balance between attraction into the electron gas around them and repulsion from the adjacent positive atomic cores. This is the electrostatic nature of metallic bonding. Its electron delocalization is also the reason that metals are such good conductors of both heat and electrical potential.
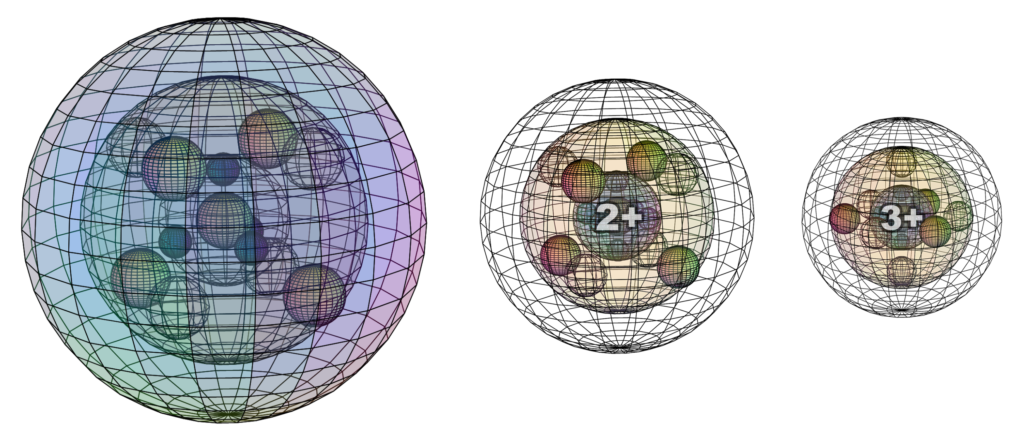
When iron interacts ionically, it usually makes the Fe2+ or the Fe3+ ion (though there are other possibilities). The Fe2+ ion forms when the atom loses its 4s2 valence electrons. The Fe3+ ion forms when the Fe2+ ion loses another electron. It is here proposed that the 3rd electron will be lost from one of the 3rd shell di-electrons, since this is the only way to retain 8-directional symmetry. The Fe3+ ion will therefore achieve the same (hexagonal bipyramidal) electron configuration we saw in chromium (Cr) and manganese (Mn), though without any 4s-electron density.
In ionic crystals — without delocalized electron density — iron ions no longer conduct electricity. They will, however, still be magnetically active because they still hold unpaired electrons.
Magnetic Properties 
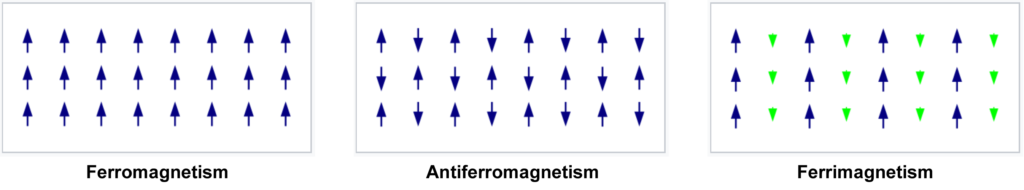
Iron (Fe), cobalt (Co), and nickel (Ni) are ferromagnetic (see below), but iron is significantly more ferromagnetic that the other two. (See Magnetism for more detail.)
UNPAIRED CORE ELECTRONS:
The presence of unpaired core electrons determines an element’s magnetic properties. While these electrons may technically be called core electrons, for the purposes of this discussion, we recognize that in a metal, if we exclude the valence “conduction” electrons, the pd-hybrid orbital electrons do become ‘valence electrons’ in a sense. Not in the sense that they can participate in chemical reactions, but in the sense that they are now in the outermost shell of the atomic cores, which are suspended in the conduction electron matrix — the 3D electron gas — of the solid metal crystal.
Unpaired core electrons are protected from reacting with other atoms. They are held in their geometry by atomic orbital constraints, they are kept from pairing up with each other by the spin and field exclusion of degeneracy, and it is proposed that they are stabilized by the presence of the 3s2 orbital ‘fundamental’ [ref].
PARAMAGNETISM:
Protected unpaired core electrons are, however, still free to respond to and orient themselves with an external magnetic field. In doing so, they cancel magnetic field with that external field (through destructive interference), which lowers energy and attracts them towards the field. The paramagnetic atom as a whole is then attracted, along with these electrons, towards the magnetic field — attracted equally to the ‘north’ as to the ‘south’ polarity. This is called paramagnetism, and it is an expected property of metals with unpaired core electrons.
When the external field is removed, the electron spins within adjacent paramagnetic atoms return to their previous, random distribution. (See paramagnetic strength trend analysis for more details.)
FERROMAGNETISM:
Ferromagnetism, however, occurs when the electrons in a substance are able to retain their internal crystalline magnetic field alignment after the external field that aligned them is removed. The solid can then act as a permanent magnet. This property therefore depends directly upon how well the unpaired electrons on adjacent atoms can link their spins and magnetic fields, thereby holding one another in alignment.
It is here proposed that there are two criteria that are required in order for the atoms of a metal crystal to achieve ferromagnetic spin bonding, and thus, a crystal-wide ferromagnetic spin resonance.
- CRYSTAL GEOMETRY: For optimal spin bonding, unpaired core electrons should have an electron domain geometry that matches the crystal unit cell geometry. We propose that the 8-directional symmetries that arise as a result of pd-hybridization may provide these.
- SUFFICIENT ORBITAL EXTENSION: Unpaired core electrons require constriction and radial extension in order to interact (via spin bonding) with similarly extended core electrons on adjacent atomic cores in a crystal. We propose that this is achieved as a result of sufficient di-electron (electron pair) repulsion within the atomic shell, along with added repulsion from the di-electrons in the 2nd shell that lie directly beneath the unpaired core electron orbitals. This criterion is therefore intimately related to the electron geometry (from criterion #1 above).
Only with both of these criteria fulfilled (see below), will a transition metal be ferromagnetic. If only one is present, or if constriction and extension are not sufficient, the element will be paramagnetic.
CRITERION #1: IRON’S CRYSTAL GEOMETRY:
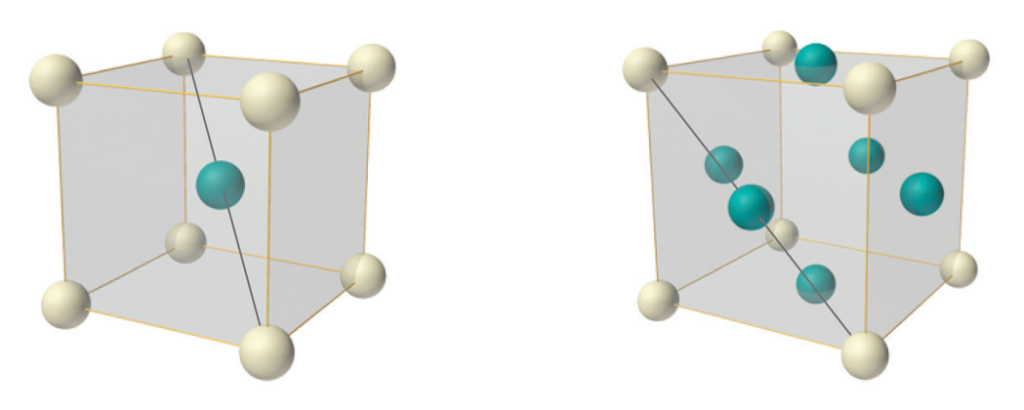
Iron is ferromagnetic below its Curie Temperature (of 770°C), and has a body-centered cubic (BCC) crystal structure. When heated past its Curie Temperature, it shifts to a face-centered cubic (FCC) crystal structure, and it also loses its ferromagnetism in favor of paramagnetism.
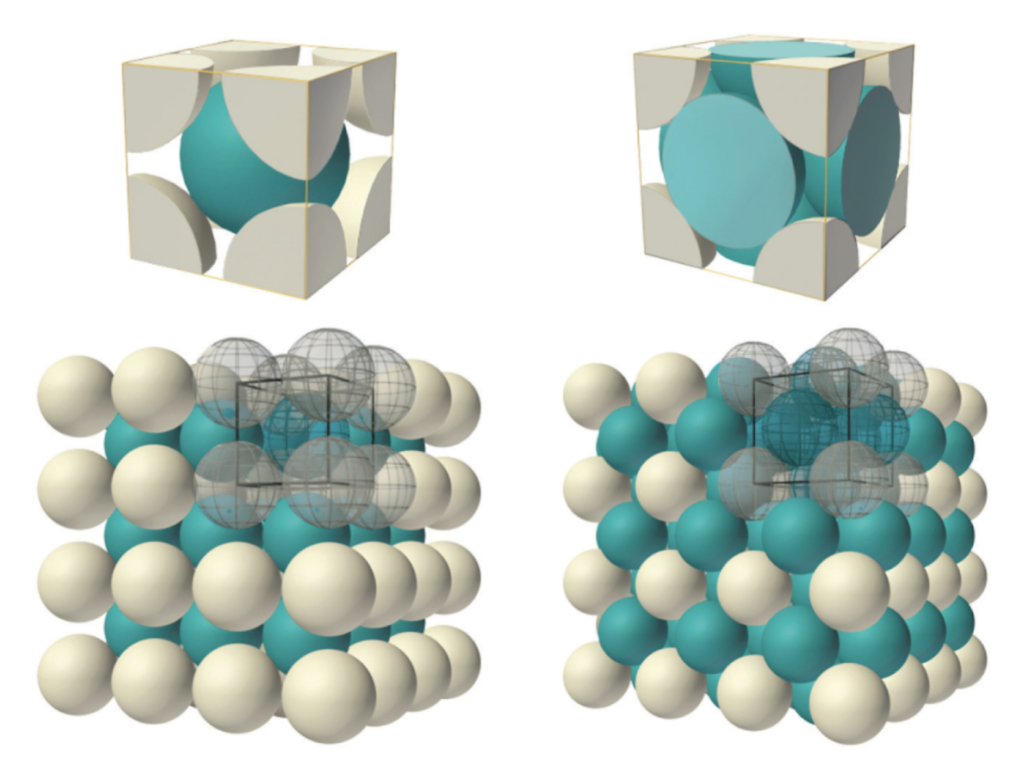
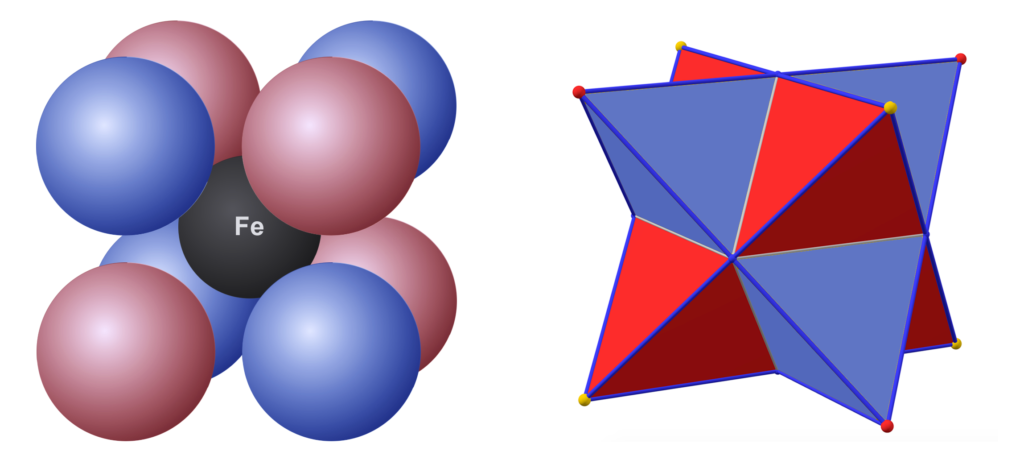
In a BCC crystal, the nearest neighbors to the blue body-centered atom in the center of the unit cell (above, left) will be the 8 white atoms arranged in a cube diagonally around it at the corners of the unit cell. This means that, in atoms with tetrahedral or double-tetrahedral (cubic) electron configurations — like that proposed here for the iron atom — the electron orbitals should align geometrically when in a BCC crystal structure. Such an alignment should, in turn, help to facilitate the proposed spin bonding (that will be discussed below).
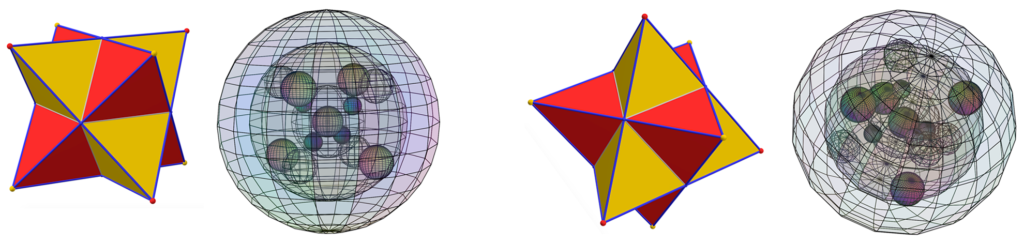
When a BCC crystal is rotated through 54.75° — half a tetrahedral angle — it can more easily be seen as two superimposed, antiparallel (and antiprismatic) tetrahedral structures that pass through every crystal vertex. Each iron atom can therefore be seen as representing the center of a dual tetrahedron. (See also fig. 3 & fig. 4 above.) The points of one tetrahedron represent the positions of the 3rd shell di-electron orbitals (the black dots in the images below), and the other tetrahedron represents the positions of the 3rd shell unpaired electron orbitals (the pink dots).
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.Through any particular iron atom, a perfect, almost diamond-like tetrahedral crystal structure of unpaired electrons runs one way, and a superimposed tetrahedral crystal structure of di-electrons runs antiparallel (and antiprismatic) to it. (Through each adjacent iron atom, the situation is reversed.) This links all of the iron atoms throughout the crystal into a single, continuous, harmonic, crystal-wide resonance of spin-bonding connection.

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object. This dual crystal structure — a network of spin-bonding unpaired electrons nested through a non-bonding, diamagnetic di-electron network — not only creates a very stable and coherent harmonic quantum state that pervades the crystal, but the intersecting di-electron network also serves to constrict the channels of that resonance further. This should serve to enhance the coherence of its connections into what we might imagine as channels of higher-density magnetic (or spin) flux. It is a harmonic resonance of both where spin is and where it is not.
It is proposed that this highly structured tetrahedral spin-bonding crystal resonance is what gives iron its powerful ferromagnetism.
CRITERION #2: SUFFICIENT ORBITAL EXTENSION:
As was proposed above, though, the alignment of spin and field is not enough to effect a ferromagnetic resonance on its own. Since these are interactions between core electrons, their influence (and connections) need to be sufficiently enhanced and extended outward from the atomic cores in order to be felt by adjacent atoms in the crystal, and to facilitate ferromagnetic spin bonding.
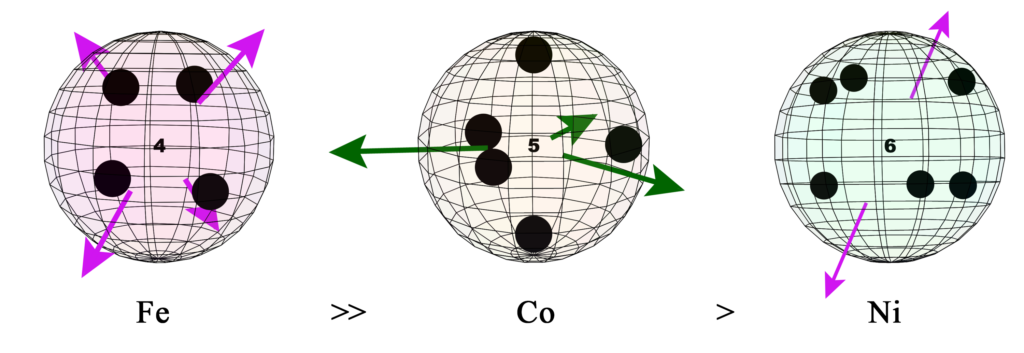
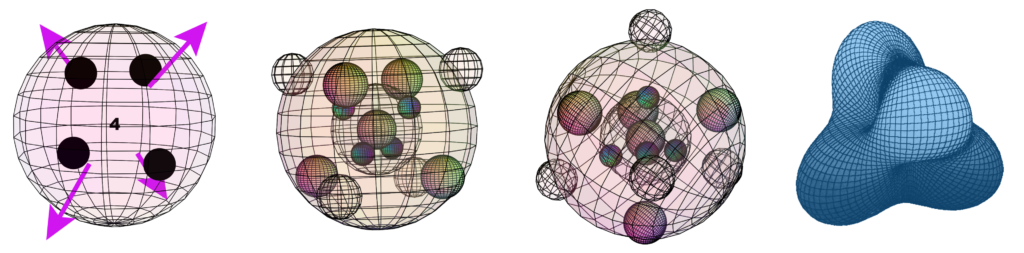
In addition to iron’s 4 tetrahedral unpaired electrons, its 3rd shell also contains 4 di-electrons. (These are depicted by large black dots in fig. 11, below right, and by the “4” in its center). Furthermore, the 4 di-electrons in the 2nd shell are aligned directly beneath the unpaired electrons of the 3rd shell.
Di-electron orbitals are larger than unpaired electron orbitals, and they have double the charge. They therefore repel unpaired electron orbitals more strongly. It has been proposed that this can not only constrict the unpaired electron orbitals but also cause them to become extended, radially outward, from the atomic core. In the case of iron, the unpaired electron orbitals are experiencing this form of constriction from eight di-electrons — 4 from beside/around them in the same shell, and 4 from beneath them in the 2nd shell.
It is proposed that the combination of all this di-electron repulsion forces the influence of the unpaired electron orbitals to be extended significantly further outward (just as a spinning cylinder, forced to become thinner, that responds by becoming longer). This extends the influence of these electrons’ spins and magnetic fields into the electron gas around the atomic cores (as shown in fig. 11, below left), and it is suggested that this is what makes electron spin interactions between adjacent crystalline iron atoms possible. [Ref]
 CLICK HERE to interact with the object (in the center).
CLICK HERE to interact with the object (in the center).(We also proposed that this concept of spin bonding explains the underlying physical mechanisms behind Pauli’s Exclusion Principle and Hund’s 2nd Rule.)
For more on the relative trend in ferromagnetic strength, see Magnetism & Magnetic Trends.
The following image is designed to evoke the idea of the extended, spin-bonding orbitals using a different analogy. While no analogy is ever a complete representation, it may be somewhat fitting in this case. Electron orbitals in the atomic quantum state have components that we might be able to think of as a type of ‘sub-quantum plasma flow state.’ The image below, of plasma tufts around an electrode, may evoke the idea of an arrangement of such extended ferromagnetic orbitals from the surface of the atomic core.
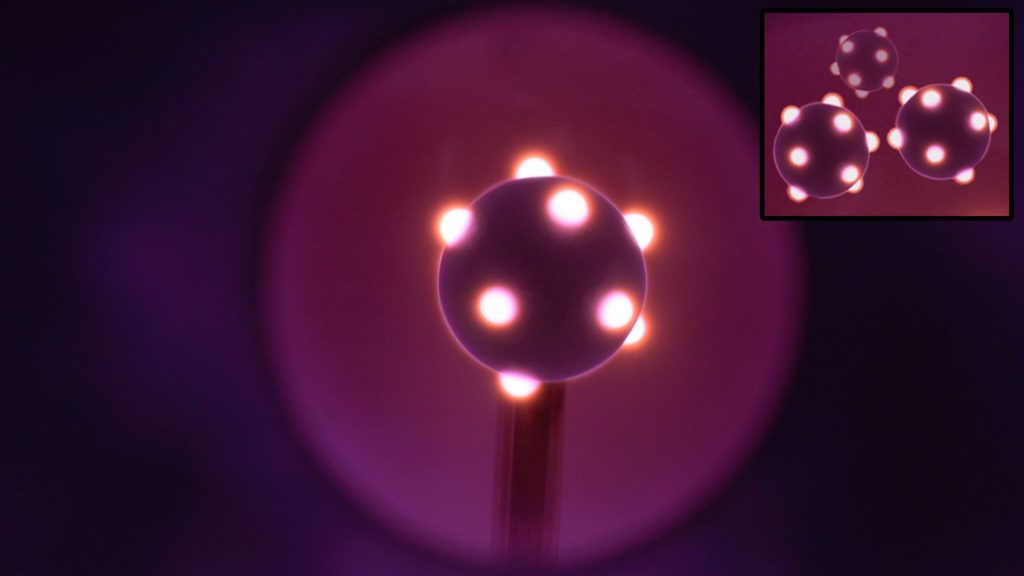
We might imagine a crystal of iron atomic cores, each like the electrode shown above, suspended in its 3-dimensional electron gas, perhaps like the violet electrode glow. In the case of iron, each positive atomic core would have 4 tufts, arranged tetrahedrally, each occupied by one of the 4 unpaired 3rd shell electrons. The remaining 4 positions of its 3rd shell cubic arrangement would be occupied by non-interacting di-electrons — also arranged tetrahedrally, though not extended, and with the di-electron orbitals separating the unpaired electron orbital tufts from one another. (The right-most image in fig. 11, above — 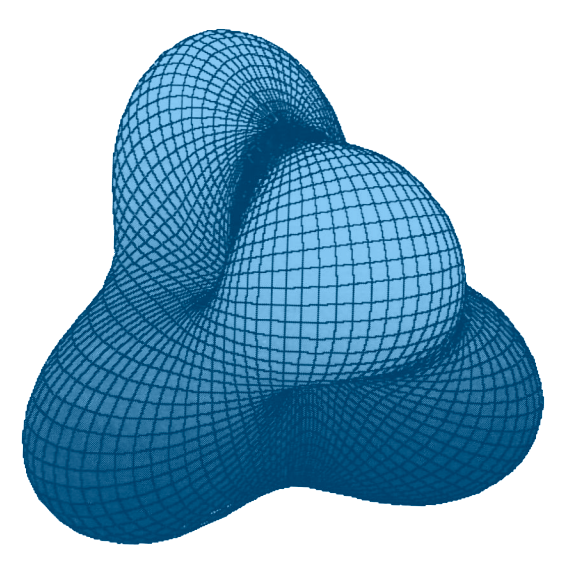 — might portray this idea nicely, though in a slightly exaggerated manner.)
— might portray this idea nicely, though in a slightly exaggerated manner.)
Curie Temperatures & Annealing
The symmetry and homogeneity of iron’s crystalline alignment is enhanced when it is annealed while being magnetized. The process of slowly cooling the iron to below its Curie Temperature (770°C), in the presence of an external magnetic field, allows the iron atoms to align with each other more perfectly and symmetrically as the metal slowly transitions from its (paramagnetic) FCC crystal form into its ferromagnetic BCC form. We might interpret this to mean that, only when thermal energy is reduced to below a certain threshold is the coherence of spin bonding strong enough to hold the crystal in a less dense but spin-optimized arrangement. Thus, as a result of its perfect geometric optimization, annealed iron has stronger ferromagnetism than regular iron.
When heated back above its Curie Temperature, iron reverts back to the denser face-centered cubic (FCC) structure, which is equivalent to hexagonal close-packing (in ‘ABC’ form). There are now no longer 8 but 12 nearest neighbors for each atom in the crystal packing, andthe optimal geometry is no longer tetrahedral but linear (as shown by the grey lines through the BCC and FCC units in fig. 10 below).
It is at this point that the iron loses its ferromagnetism. (It will, however, still remain strongly paramagnetic!)
It is proposed that this loss of ferromagnetism occurs because the change in packing and density causes the tetrahedral hybrid orbitals to no longer align perfectly with those of their crystalline neighbors. This should disrupt the crystal-wide spin-bonding coherence. The iron will remain paramagnetic because the ‘mis-aligned’ unpaired electron orbitals will still respond eagerly to the field cancellation offered by an external magnetic field.
RELATIVE STRENGTH & CURIE TEMPERATURE: Iron is by far the most strongly ferromagnetic of the three metals, yet cobalt has a higher Curie Temperature. This means that, even though iron is more strongly magnetic, cobalt (Co) can hang on to its magnetization to a much higher temperature. That must mean its ferromagnetic spin resonance is stronger than iron’s. How might we explain this?
Iron is by far the most strongly ferromagnetic of the three metals, yet cobalt has a higher Curie Temperature. This means that, even though iron is more strongly magnetic, cobalt (Co) can hang on to its magnetization to a much higher temperature. That must mean its ferromagnetic spin resonance is stronger than iron’s. How might we explain this?
According to the present proposed model, we might speculate that the reason for this is as follows. Cobalt contains three instances of ferromagnetic spin bonding (FSB) per atom, and the resonance is essentially two-dimensional, in the hexagonal crystal layers. Iron contains four instances of FSB per atom, and the resonance is three-dimensional and perfectly tetrahedral throughout the lattice. This causes iron to have a far more significant ferromagnetic spin resonance throughout its crystal, making it more strongly ferromagnetic than cobalt.
However, each of cobalt’s three instance of FSB involves 3 electrons holding each other in spin resonance. Each of iron’s four instances involve only 2-electron resonances. As such, cobalt’s spin bonding resonances are stronger and will therefore be able to withstand higher temperatures without thermalization disrupting them out of resonance. It is therefore proposed that this is what gives cobalt a higher Curie Temperature than iron, in spite of its weaker ferromagnetic strength.
Other Types Of Magnetism
ANTIFERROMAGNETISM:
Antiferromagnetism is a state in which the unpaired electron spins on adjacent atoms in the metallic crystal are anti-parallel to one another. This creates a net spin-zero state for the crystal as a whole, and it will therefore not exert an external magnetic force.
It is proposed that antiferromagnetism is not a form of ferromagnetism, but rather a ‘local’ resonance resulting from an electron-gas coupling.
For more information, see chromium (Cr) or Magnetism & Magnetic Trends.
OTHER TYPES OF MAGNETISM:
There are also other types of magnetism, for example ferrimagnetism, altermagnetism, and an effect known as ‘spin glass‘ involving neodymium (Nd), but these will not be investigated here.
For more information, see Magnetism & Magnetic Trends.
References:![]() See DeMystifySci Podcast: The Strange Behavior Of Humans And Magnets for a brief video discussion of this concept.
See DeMystifySci Podcast: The Strange Behavior Of Humans And Magnets for a brief video discussion of this concept.![]() A. Benn, J.G. Williamson, ‘pd-Hybridization And The Electron Geometry Of Fluorine, Neon And Iron’, Quicycle Journal (2024)
A. Benn, J.G. Williamson, ‘pd-Hybridization And The Electron Geometry Of Fluorine, Neon And Iron’, Quicycle Journal (2024)![]() J.G. Williamson, A. Benn, M. Rudolph, ‘Quantum Spin Coherence In 4 Derived 3-Spaces’, Quicycle Journal (2022)
J.G. Williamson, A. Benn, M. Rudolph, ‘Quantum Spin Coherence In 4 Derived 3-Spaces’, Quicycle Journal (2022)
PARAMAGNETIC 3d METALS: Scandium, Titanium, Vanadium, Chromium (also antiferromagnetic), Manganese
OTHER FERROMAGNETIC 3d METALS: Iron, Cobalt, Nickel
DIAMAGNETIC 3d METALS: Copper, Zinc
RETURN to the Periodic Table
