
RETURN to Periodic Table
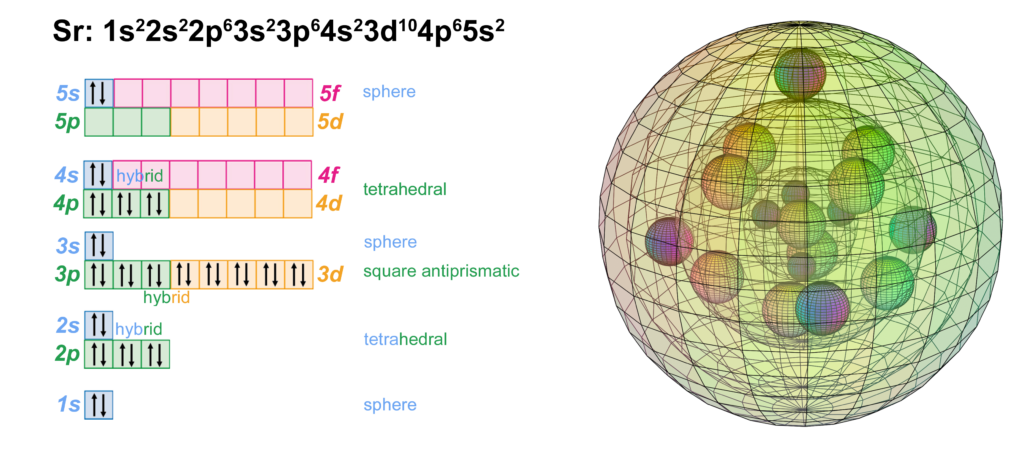
Strontium has an s-orbital di-electron in the 5th shell. It has the same electron configuration as calcium, but with four full shells within that have the identical configuration to krypton. Being one shell larger than calcium, its valence electrons are less well bound. This causes it to have a lower ionization energy and be more reactive and more soluble than calcium, allowing it to form the 2+ ion more readily.
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectRETURN to the Periodic Table
OTHER GROUP II ELEMENTS: Beryllium, Magnesium, Calcium, Strontium, Barium
