
RETURN to Periodic Table
Aluminium (or Aluminum) is the 13th element on the periodic table. It has 13 protons and 14 neutrons in the nucleus, giving it a mass of 27 amu, and it has 13 electrons enveloping the nucleus.
Electron Shell
Aluminium has interesting chemistry and bears similarities to several different elements. It lies in Group III beneath boron, and with a 3p1 electron configuration, it is similar to boron’s 2p1 configuration. This implies that it will form a triangular (sp2 trigonal planar) geometry. It also has similarities to beryllium and can form bonds of a covalent nature. However, since aluminium is larger than boron, its valence electrons fill a larger volume and are therefore less coherent as a single quantum system. This makes aluminium more metallic in nature than the semi-metallic boron above it. (The wireframe indicates the boundary of the n=3 shell.)
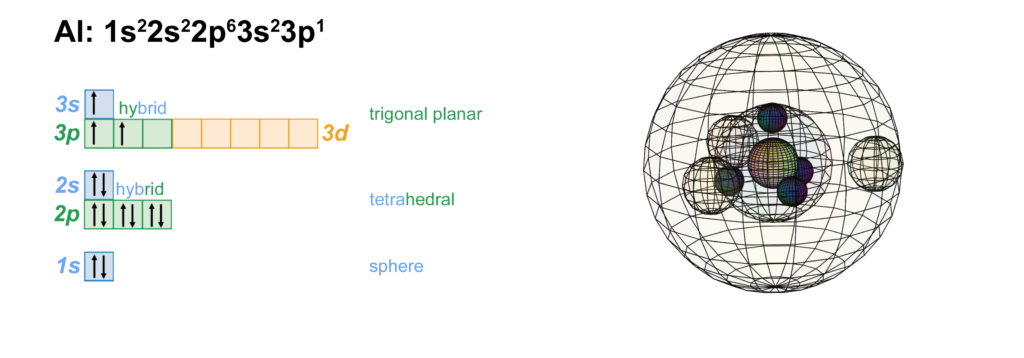
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell orbitals themselves will be more like three longitudinal sections. The entire shell will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals, where electron density will be lowest (though not zero).
Each of these three hybrid orbitals contains one electron. Like boron’s configuration, this arrangement is symmetrical in the equatorial plane but does not have equivalent symmetry in all directions.
Bonding & ion formation
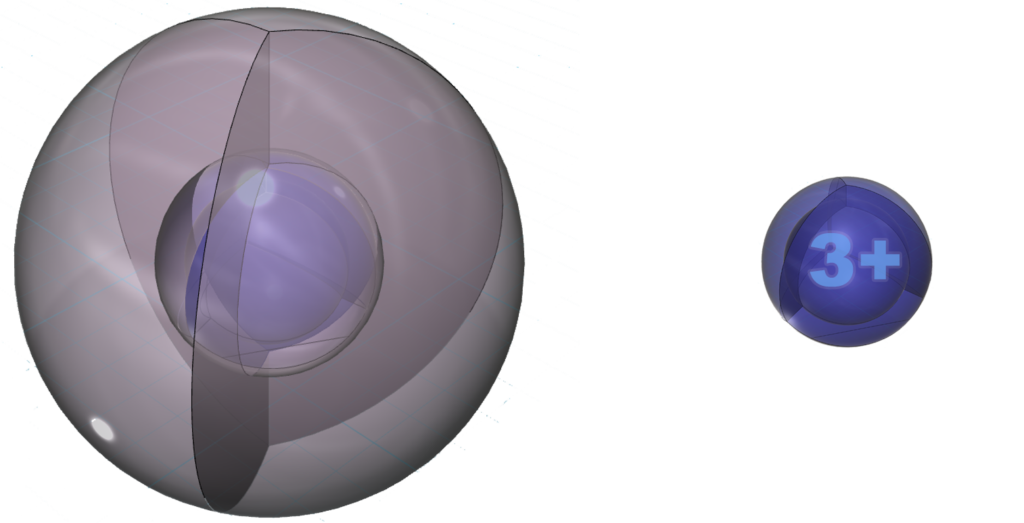
Aluminum will bond with other metal atoms via a metallic bond, and with non-metals via an ionic bond in which it loses its three valence electrons. It will lose all three at once in order to reach the stability of a full 2nd shell. This is the same electron configuration as the 2s22p6 noble gas configuration of neon — a multi-di-electron state with two concentric full shells. That is why aluminium forms a 3+ ionic state, as in aluminum oxide (Al2O3).
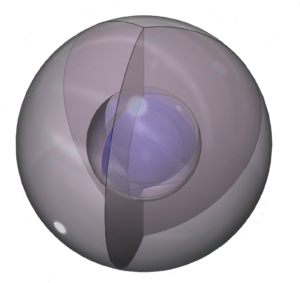
The positive ion is much smaller than the neutral atom because protons now outnumber electrons by three. This results in a much higher effective nuclear charge — a higher average attraction by the nucleus on each electron, which shrinks the size of the electron shell as it is attracted inward with more force.
Some uses & properties
Unlike most other small ions, aluminium ions do not play a biologically useful role. In fact, given their small size and high charge, Al3+ ions are toxic to most organisms.
Aluminium occurs in nature in a bonded, crystalline form. Reducing it to pure metal is done via electrolysis, and uses a large amount of electricity because it requires 3 electrons for every atom of aluminium to turn Al3+ ions into Al0 metal. One of the key uses of this light and strong metal is in the manufacture of aircraft. The first aircraft engine, built by the Wright brothers, was made out of aluminium specifically due to its low density.
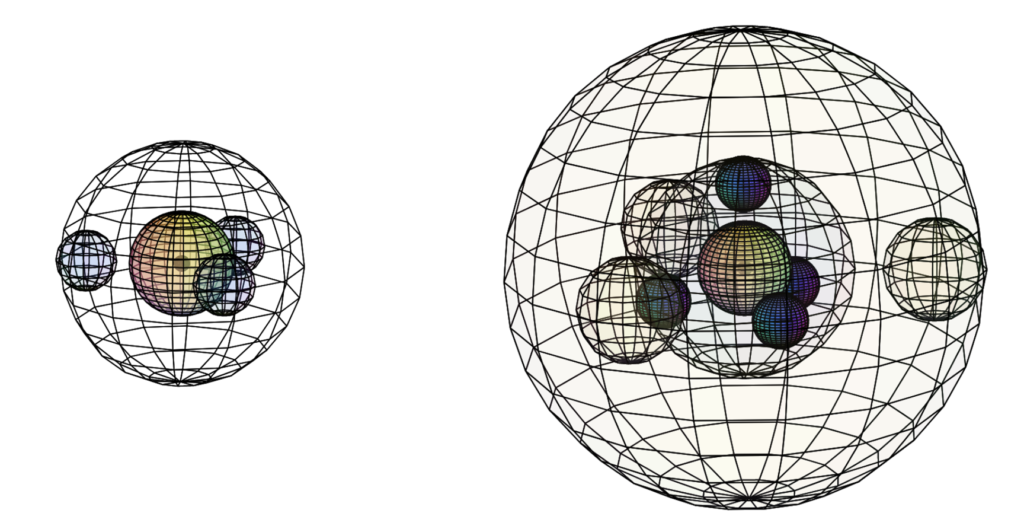
When we compare aluminium to boron (B), we see a significant size difference (see below). We also see a significant difference between the magnetic susceptibility of aluminium and the other Group III elements, since aluminium is paramagnetic (χm=+16.5) while the others are diamagnetic. Aluminium’s paramagnetic strength is also very similar to that of sodium (Na) (χm=+16).
This might suggest that, in its crystalline metallic state, aluminium also delocalizes only one of its three valence electrons, perhaps allowing the remaining two 3s-electrons to retain some of their preferred spherical di-electron character, stabilizing the 3rd shell with spherical electron density. (This state would not be possible if aluminium were bonding with any other element. Differences in size, effective nuclear charge, and electronegativity would coax the aluminium atom into its normal trivalent configuration.)
If this speculation is correct, it might account for the following observations:
- aluminium has a magnetic susceptibility value similar to sodium (Na).
- aluminium has a 2nd ionization energy whose increase is 50% higher than the elements on either side of it (Mg & Si). This suggests that, compared to magnesium and silicon, it is uncharacteristically difficult to remove (or delocalize) aluminium’s 2nd (and 3rd) valence electron.
- aluminium is a soft metal. It seems reasonable that the presence of a larger 1+ metallic core with a single delocalized electron per atom should result in a lower density metal with weaker metallic bonds than a smaller 3+ metallic core with three delocalized electrons per atom.
- If aluminium only delocalizes one electron per atom — like copper (Cu), silver (Ag), and gold (Au) — it might account for the fact that aluminium is the 4th best electrical conductor, after silver, copper, and gold.
… and it might have a topology like this:
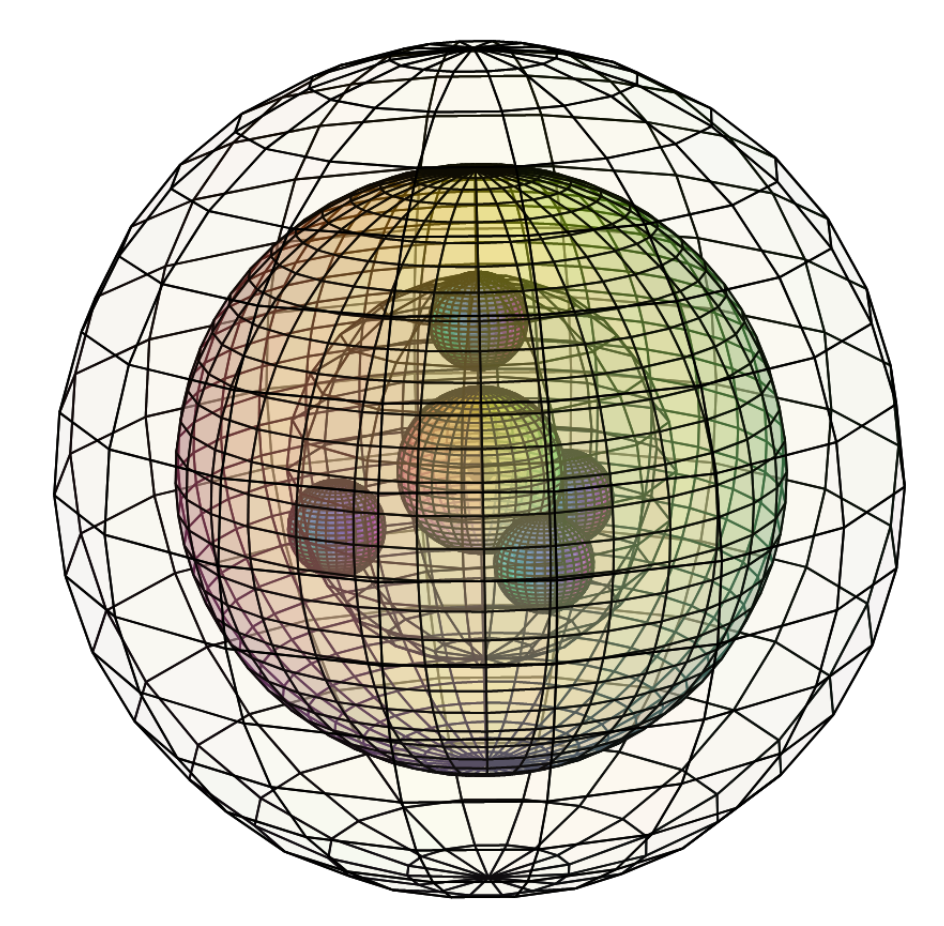
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectBoron’s diamagnetism (χm=–6.7), on the other hand, would appear to emerge from the fact that it usually makes covalent bonds, in which all of its electrons are paired.
RETURN to the Periodic Table
