
RETURN to Periodic Table
Chlorine is the 17th element on the periodic table. It has 17 protons and (mostly) 18 neutrons in the nucleus, giving it a mass of 35 amu, and it has 17 electrons enveloping the nucleus.
Electron Shell
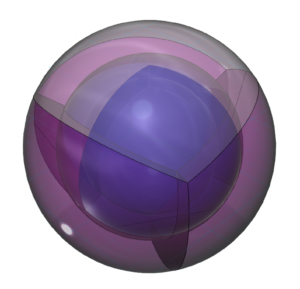
Chlorine has the same electron configuration as fluorine, just one shell larger. This allows chlorine a broader palette of chemical reactions, given the presence of a d-orbital. Like sulfur, this gives rise to more possible molecular geometries. Chlorine can sustain multiply bonded atoms, but it takes bonding atoms with stronger electronegativity than chlorine in order to draw it into multiple bonds. This limits to list of contenders essentially to oxygen and fluorine, as we see in the chlorate (ClO3–) ion. (The wireframe indicates the boundary of the n=3 shell.)

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell orbitals themselves will be more like spherical tetrahedra. The entire shell will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals, where electron density will be lowest (though not zero). Like argon, chlorine features two nested spherical tetrahedra, the inner 2nd shell comprising 4 di-electrons, the outer 3rd shell comprising 3 di-electrons and 1 single electron. In this case the three orbitals containing the di-electrons (dark pink) will occupy slightly more volume than the one containing an unpaired electron.
Bonding & ion formation
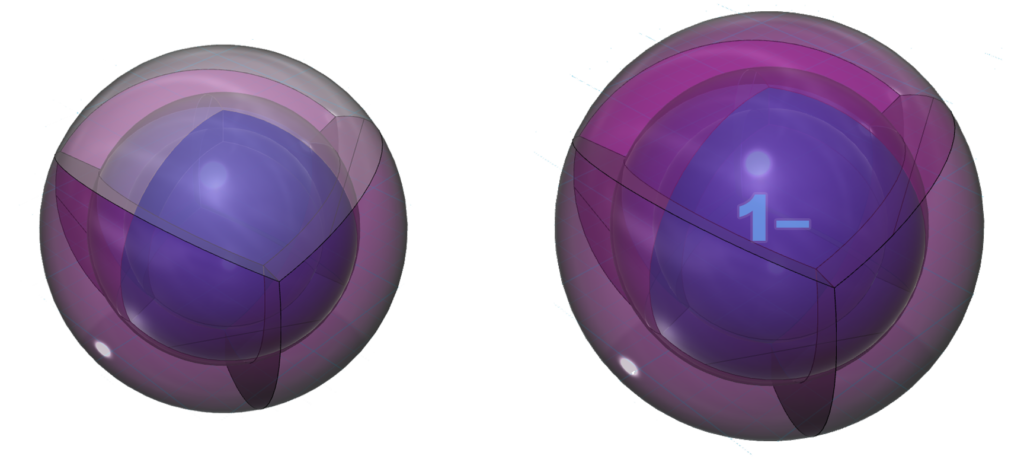
Chlorine is keen to obtain an extra electron to fill its third shell and it can bond with many atoms on the periodic table. Chlorine can make one or more covalent bonds or gain an electron in ionic interaction in order to reach the stability of the 3s23p6 noble gas configuration of argon, which is a multi-di-electron state with three concentric full shells. That is why chlorine forms a 1– ionic state. The negative ion is larger than its neutral version because electrons now outnumber protons. This results in a lower effective nuclear charge — a lower average attraction by the nucleus on each electron.
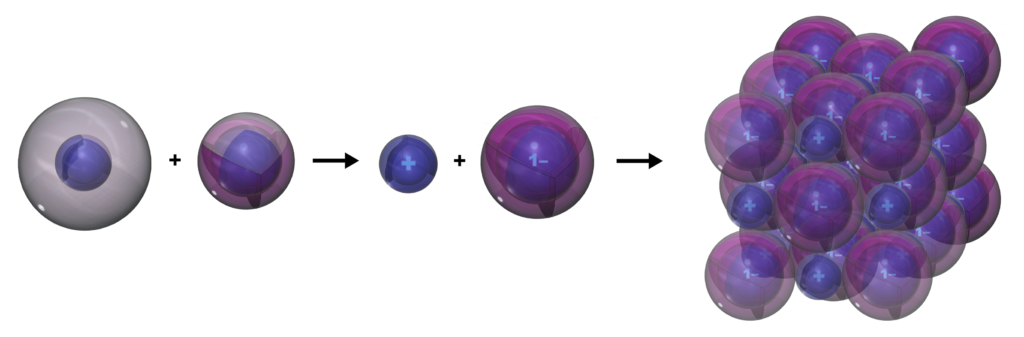
Salt
When sodium and chlorine interact, sodium gives the electron it wants to lose to chlorine, which is keen to gain it. This forms both atoms into their ions and allows both to achieve full shell configurations. The ions can then stick to each other because of their opposite charges, forming sodium chloride (NaCl) crystals. This process is called ionic bonding, and it occurs between a metal (from the left side of the periodic table) and a non-metal (from the right side). The term “salt” can also be used to apply to any ionic crystal.
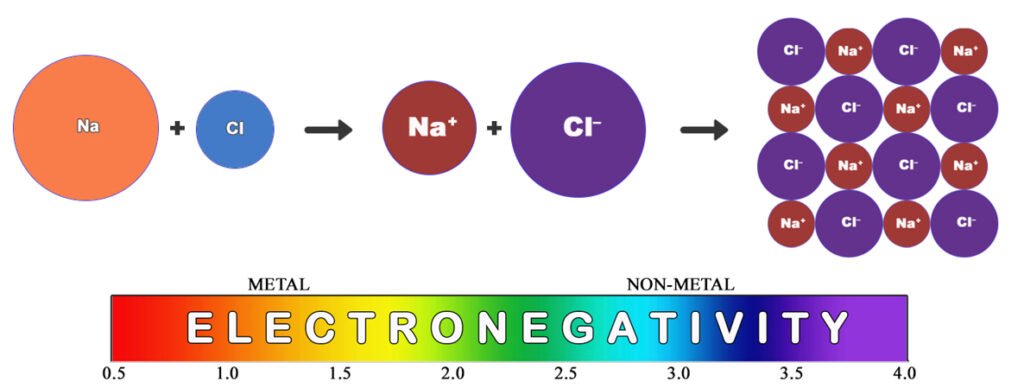
Below is another visualization of the formation of sodium chloride from the point of view of electronegativity. The electrons in a bond will always be pulled more strongly towards the atom that is closer to the violet end of the electronegativity spectrum. In this case, there is such a great difference between how strongly they hold their electrons that the chlorine will be able to strip the sodium’s electron completely away. This clarifies why the two ions form, as opposed to them still sharing the electrons (as occurs in water).
Sodium chloride (NaCl) dissolves in water because the polar H2O molecules and the ions in the crystal attract each other. The water molecules can therefore tug ions off the crystal and still satisfy the ion’s desire to attract their opposite polarity. As each ion leaves the crystal, it becomes hydrated — surrounded by water molecules.
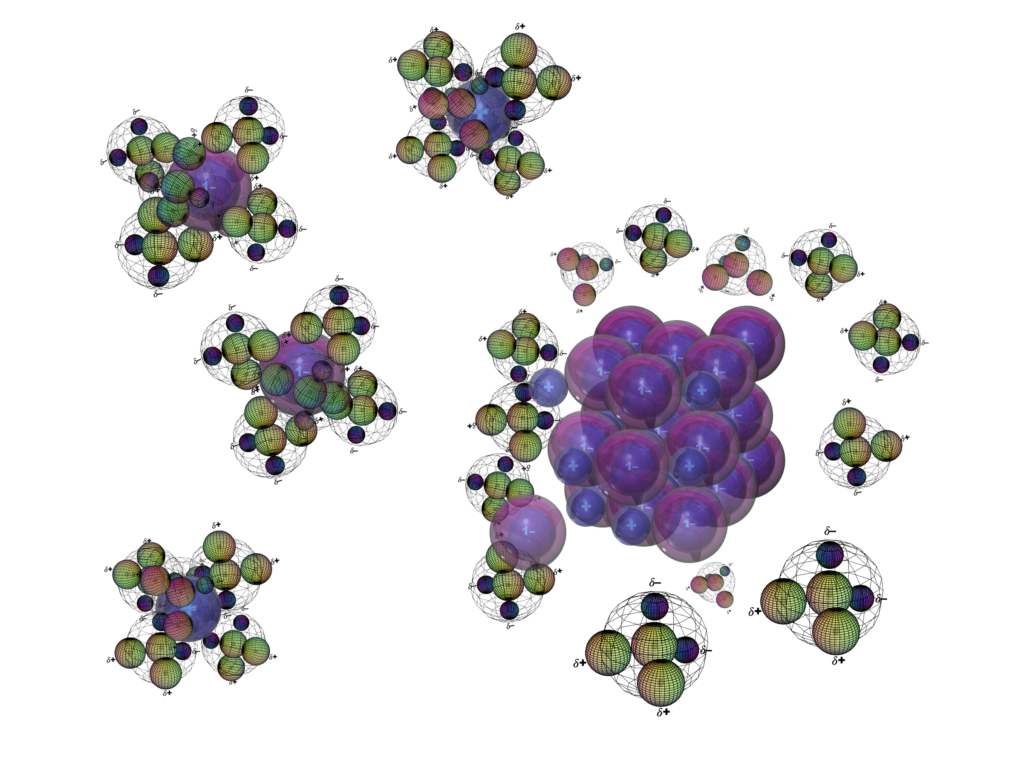
When the water is allowed to evaporate from the salt solution, the ions become increasingly exposed to one another, and the solid crystals re-form due to electrostatic attraction.
Although the ocean contains many different ions and just about every element on the periodic table (in trace amounts), it is mainly made up of water (H2O) and sodium chloride (NaCl). Of these four elements, the mass of sea water is made up of about 86% oxygen, 11% hydrogen, 1.9% chloride, and 1.1% sodium.
RETURN to the Periodic Table
OTHER GROUP VII HALOGENS: Fluorine, Chlorine, Bromine, Iodine
