
RETURN to Periodic Table
Bromine is the fifth element with electrons in the 4p orbital. It has the same electron configuration as chlorine, just one shell larger. Like chlorine, bromine can support multiple possible molecular geometries, and can sustain multiply bonded atoms (with stronger electronegativity than bromine). (The wireframe indicates the boundary of the n=4 shell.)
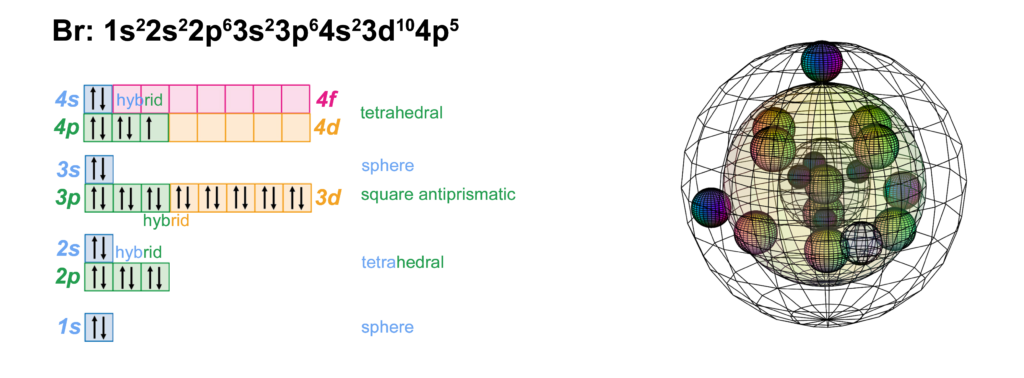
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectBonding & ion formation
Bromine is keen to obtain an extra electron to fill its fourth shell and it can bond with many atoms on the periodic table. Bromine can make one or more covalent bonds or gain an electron in ionic interaction in order to reach the stability of the 4s24p6 noble gas configuration of krypton, which is a multi-di-electron state with four concentric full shells. That is why bromine forms a 1– ionic state. The negative ion is larger than its neutral version because electrons now outnumber protons. This results in a lower effective nuclear charge — a lower average attraction by the nucleus on each electron.
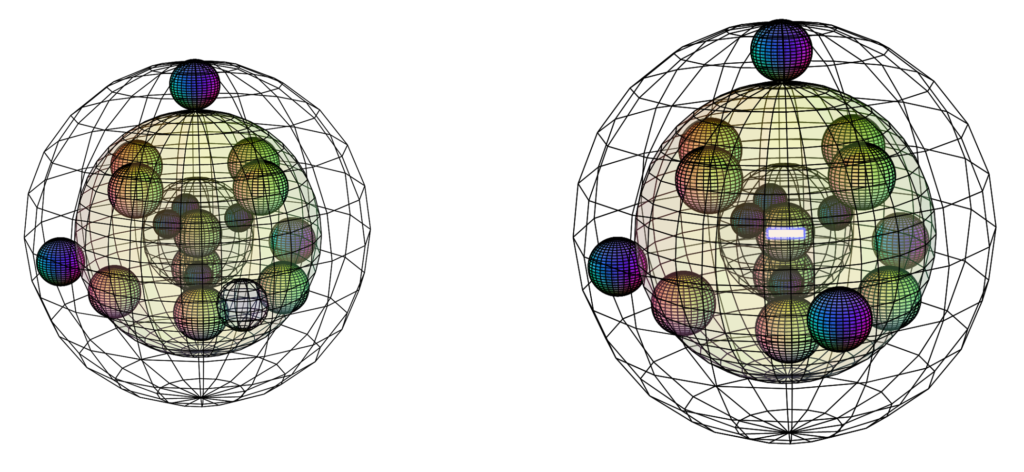
RETURN to the Periodic Table
OTHER GROUP VII HALOGENS: Fluorine, Chlorine, Bromine, Iodine
