
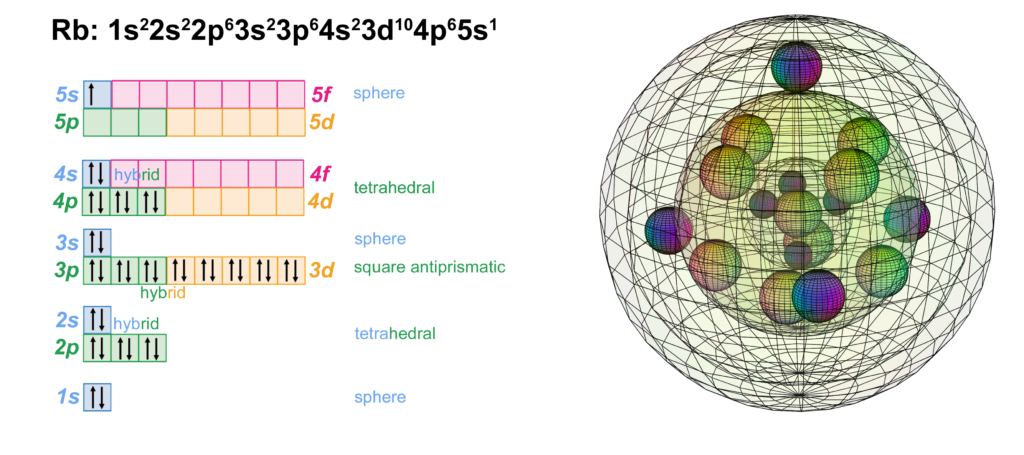
RETURN to Periodic Table
Rubidium has one electron in the 5th shell. It has the same electron configuration as potassium, but with four full shells within that have the identical configuration to krypton. Being one shell larger than potassium, rubidium has a lower ionization energy and is therefore more reactive, forming the 1+ ion more readily. Pure rubidium metal will self-ignite in air as it reacts with (and donates its valence electron to) oxygen.
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectIon formation
With a lower ionization energy than potassium, Rubidium will give up its valence electron more eagerly than potassium in an ionic interaction, in order to reach the stability of the 4s24p6 noble gas configuration of krypton, which is a multi-di-electron state with four concentric full shells. That is why rubidium forms a 1+ ionic state so violently.
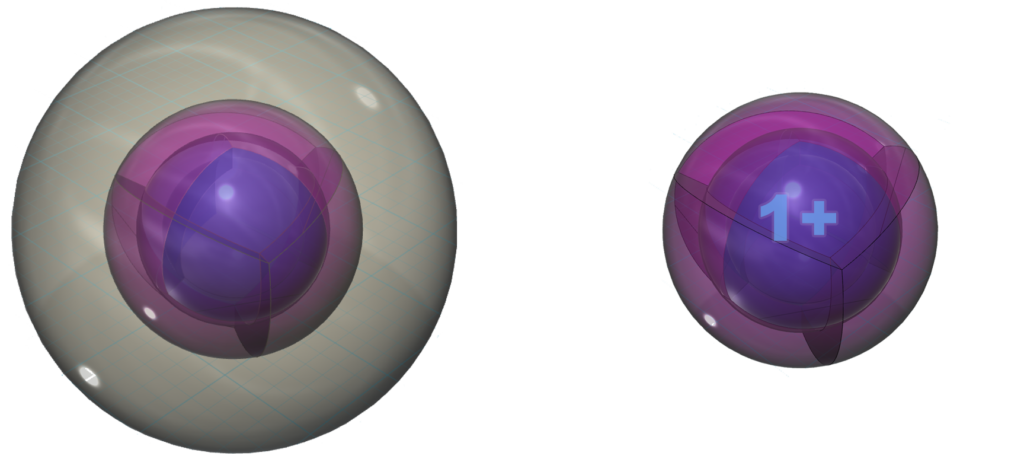
Pure rubidium metal can react in the presence of oxygen gas and spontaneously ignite as it donates its valence electron to oxygen. Rubidium burns with a deep-red flame, hence its name.
A fun video showing rubidium metal and its reactivity can be found HERE.
RETURN to the Periodic Table
OTHER GROUP I ELEMENTS: Lithium, Sodium, Potassium, Rubidium, Cesium
