
RETURN to Periodic Table
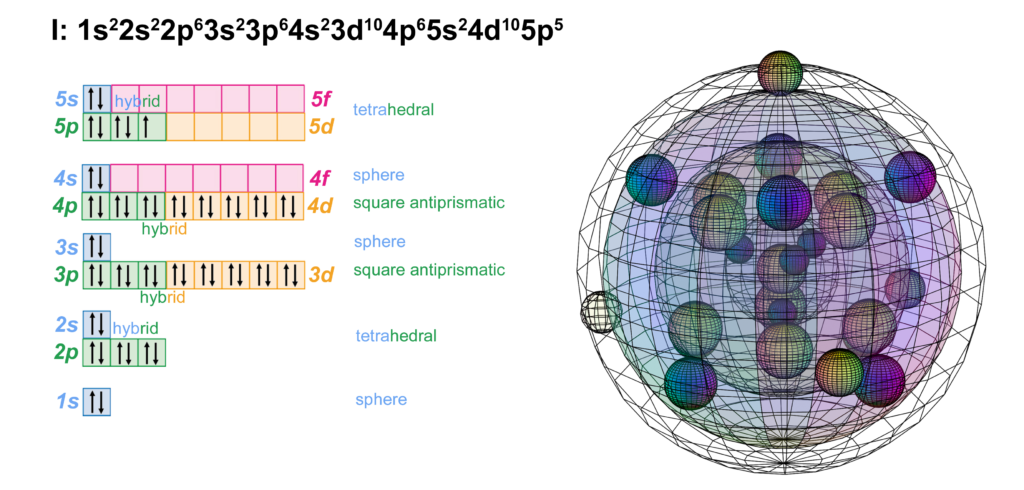
Iodine is the fifth element with electrons in the 5p orbital. It has the same electron configuration as bromine, just one shell larger. Like chlorine and bromine, iodine can support multiple possible molecular geometries, and can sustain multiply bonded atoms (with stronger electronegativity than iodine). (The wireframe indicates the boundary of the n=5 shell.)
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectBonding & ion formation
Iodine is keen to obtain an extra electron to fill its fifth shell and it can bond with many atoms on the periodic table. Iodine can make one or more covalent bonds or gain an electron in ionic interaction in order to reach the stability of the 5s25p6 noble gas configuration of xenon, which is a multi-di-electron state with five concentric full shells. That is why iodine forms a 1– ionic state. The negative ion is larger than its neutral version because electrons now outnumber protons. This results in a lower effective nuclear charge — a lower average attraction by the nucleus on each electron.
RETURN to the Periodic Table
OTHER GROUP VII HALOGENS: Fluorine, Chlorine, Bromine, Iodine
