
Here is a list of chemistry definitions that may be useful.
(See Glossary for terms that are not specifically related to chemistry.)
A – B – C – D – E – F – H – I – L – M – N – O – P – Q – R – S – V
A
Acid:
An acid is a substance that releases hydrogen (H+) ions into solution when placed in water. The stronger an acid, the more H+ ions it produces in solution. Hydrochloric acid (HCl) is a strong acid because it releases all of its hydrogen into solution as H+ ions. A weak acid, like vinegar (HC2H3O2), only releases a small percentage. An H+ ion is an exposed proton, which is why strong acids can be so corrosive. Each H+ ion is desperately seeking an electron in which to clothe its bare proton nucleus. It does so by taking an electron from another substance around it, which results in a (sometimes violent) chemical reaction.
Alkali:
See Base
Atomic mass unit (amu):
For convenience, the mass of atoms is usually considered in terms of the mass of the proton (or neutron). One amu is the same as the mass of a single proton, which is 1.67×10−27 kg. This is not a very convenient number to work with, so, instead of saying that a helium atom has a mass of 6.69×10−27 kg, it is easier to say that helium has a mass of 4 amu.
Atomic Radius:
This refers to the size of the atom by referring to the distance from its center to the “edge” of its electron cloud. The “bonding atomic radius” is slightly shorter than the radius of the neutral atom because, when atoms bond, their electron clouds tend to overlap. This makes the radius shorter at that place, and it is calculated by taking half of the bond length — the distance from the nucleus of one atom to the nucleus of the other.
B
Base:
A base (or alkali) is a substance that attracts and bonds with hydrogen (H+) ions in solution. Most bases are therefore negative ions, like hydroxide (OH–) or hydride (H–), or else they contain atoms with exposed di-electrons (lone pairs), like nitrogen, because these negative charge centers strongly attract positively charged H+ ions.
C
Curie Temperature (Tc):
The temperature at which a ferromagnetic metal loses its permanent magnetization. This occurs because there is so much thermal entropy at this temperature that the spin orientations between unpaired electrons on adjacent atoms are disrupted.
D
Diamagnetism:
This is when a substance is repelled from a magnetic field because it contains paired electrons. These paired electrons are in a state of field cancellation with one another. The presence of an external magnetic field will disrupt the coherence of this di-electron state, raising its energy. This causes it to repel away from the field in search of a lower energy state. Copper (Cu) and zinc (Zn) are diamagnetic.
Di-electron:
A pair of electrons of opposite spin that have completely superimposed upon one another. This yields a single, coherent, quantum state which brings their magnetic fields into equal and opposite orientations at every point within the di-electron, facilitating almost total magnetic field cancellation. It also facilitates a cancellation of the equal and opposite quantum spins, converting two fermions (with spin 1/2) to one boson (with spin 0). This results in a highly stable and favorable state, in spite of the like-charge electric field repulsion, because it allows for a significant lowering of energy. Examples of di-electrons include the 1s2 shell in helium (He), the covalent bond in the hydrogen molecule (H2), the “lone pair” on the nitrogen atom (N), and the superconducting Cooper pair. For more on electrons, see Understanding electrons.
E
Effective Nuclear Charge (Zeff):
The protons in the nucleus attract the electrons in the electron cloud around it. The more protons in the nucleus and the more electrons in the orbitals, the more strongly the two are attracted towards each other. This shrinks the size of the atom. In general, effective nuclear charge increases as we move to the right in a row on the periodic table, since we are adding both protons and electrons when we move to the next element. This means atoms get smaller as we move to the right in a row on the periodic table. In general, effective nuclear charge decreases as we move down a column (group) on the periodic table, since we are adding electron shells. The outer electrons are further from the nucleus, which both decreases their attraction to it, and the new shell adds diameter to the atom.
Electron (e–):
One of the three subatomic particles out of which all atoms are made. (The other two are the proton and the neutron.) An electron is negatively charged and highly stable. Contrary to popular misconception, it is not a point particle but it has a sub-structure that gives rise to its properties. It is made of a single photon of light making two revolutions per wavelength,. An electron is thus a self-confined knot of concentrated light energy traveling around itself at the speed of light. It has a toroidal (donut-shaped) sub-structure in (momentum) space, but the charge field of an isolated electron (or an s-orbital electron around a hydrogen or helium atom) manifests as a sphere. As a result of the geometry of this double-loop torus, the photon’s negative electric field is pointing outwards at all times, which is what gives the electron its negative charge. An electron is a fermion and has a left-handed spin of S=½, a charge of C=-1.6×10-19 Coulombs, and a mass-energy content of 511 keV. For more on electrons, see Understanding electrons  .
.
Electron affinity:
This is a measure of how much an atom wants to accept another electron. It is a measure of how much an added electron would stabilize the atom, so it is quantified by how much energy is released when an electron is added. The more negative the energy value, the more the atom wants another electron. Atoms like chlorine (Cl) and fluorine (F) have the largest negative values. If the value is positive, it indicates that the atom’s orbital resonance is so stable that an added electron would be unfavorable and would increase energy. Examples include noble gases, like neon (Ne), or atoms with a full orbital, like zinc (Zn).
Electronegativity (χe):
When two atoms bond, electronegativity is a measure of how strongly an atom’s nucleus pulls the bonding di-electron toward itself. When there is an electronegativity difference between the two bonding atoms, the more electronegative atom will pull electron density more strongly, resulting in an imbalance in the electron sharing. This results in a polar bond. In general, the smaller the atom, the higher its electronegativity. This is due to effective nuclear charge. (See above.)
F
Ferromagnetism:
This is when a substance can retain its internal magnetic field alignment and therefore act as a permanent magnet. When exposed to an external magnetic field, the unpaired electrons throughout the substance align with the field, and when the field is removed, the electrons remain in alignment with one another. Iron (Fe), cobalt (Co), and nickel (Ni) are ferromagnetic.
H
Hybridization:
When different orbitals in the same shell, like an s-orbital and a p-orbital, resonate together since they are occupying the same volume of space. This combination of their electron densities changes their shape in order to achieve greater symmetry and stability.
I
Ion:
An atom that has either lost or gained one or more electrons. This means there is no longer a balance between the positive protons in the nucleus and the negative electrons enveloping it. The atom therefore has an overall charge. If it lost electrons it will be a positive ion (also known as a cation). If it gained electrons it will be a negative ion (also known as an anion). In nature, crystals (like salt or quartz) are made of ions. While the term can refer to either positive or negative ions, it is often used to refer to positive ions, as in the case of cosmic rays, for example. Electromagnetic radiation that has enough energy to knock electrons free from atoms is called ionizing radiation. This also makes it hazardous to biological tissue, since changing the charge of a molecule in the body will affect the chemical role it plays.
Ionization Energy:
This is the amount of energy needed to remove an electron from an atom, to overcome the attraction from its nucleus. (An analogy might be giving a rocket enough thrust to escape a planet’s gravity.) The higher the ionization energy, the harder it is to remove the electron. The lower the ionization energy, the easier it is to remove the electron. The most reactive elements will be those with either low or high ionization energies. This is because they will either be eager to donate an electron or steal an electron (respectively) in a chemical reaction. In general, ionization energy increases as we move to the right in a row on the periodic table, since effective nuclear charge is increasing (see above). In general, ionization energy decreases as we move down a column (Group) on the periodic table, since the valence electrons are further from the nucleus and therefore less strongly attracted to it.
Isotope:
An element is defined by how many protons it contains in its nucleus. But it can have varying numbers of neutrons, which changes its mass but does not affect its charge because neutrons are neutral. By way of example, the three hydrogen isotopes are protium  , deuterium
, deuterium  , and tritium
, and tritium  .
.
L
London Dispersion Forces:
When two atoms or molecules approach each other, their electron clouds experience mutual repulsion. This causes the electrons on both of them to shift slightly so that a weak polarity is induced. If there are enough electrons present (because the atoms are larger), this polarity can become quite meaningful.
M
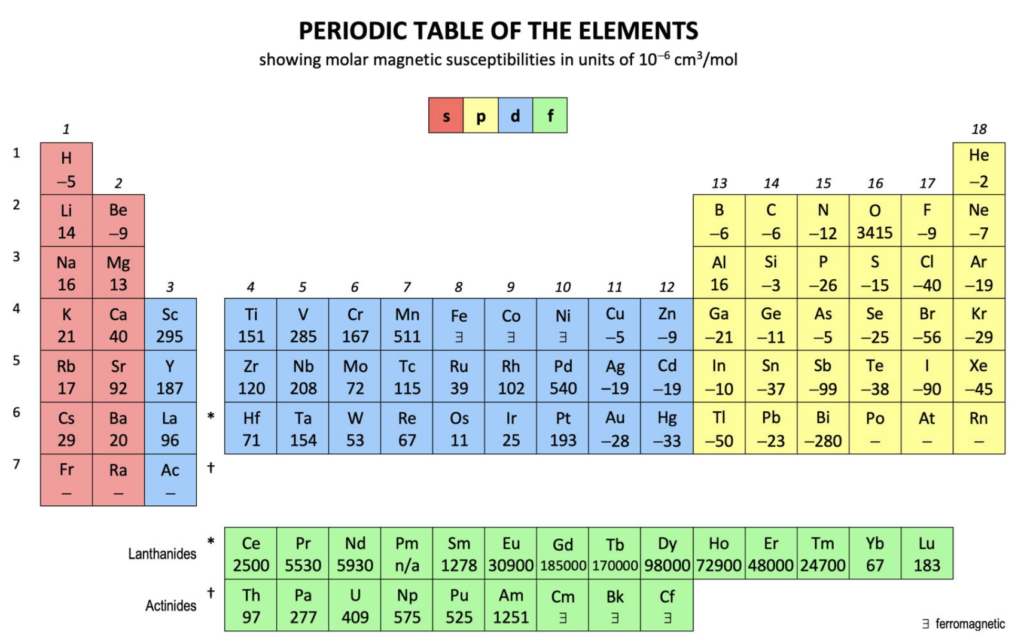
Magnetic Susceptibility (χm):
This is a measure of the extent to which a substance ‘becomes magnetized’ when exposed to an external magnetic field, and thus, resonates together with the field. It is therefore also a measure of how strongly an element will be attracted (or repelled) by an external magnetic field.
A negative value means the substance is diamagnetic — it has all of its electrons paired and it repels from an external magnetic field. A positive value means the element is paramagnetic — it has unpaired electrons and it is attracted into an external magnetic field.
N
Neel Temperature (TN):
The temperature at which the electrons in an antiferromagnetic metal lose their alternating spin alignments and they become paramagnetic. This means they will be able to align in the same direction as an external magnetic field. It occurs because there is enough thermal entropy at this temperature that the spin orientations between unpaired electrons on adjacent atoms can be disrupted.
Neutron (n0):
One of the three subatomic particles out of which all atoms are made. (The other two are the proton and the electron.) A neutron carries no overall charge and, like a proton, is almost 2,000 times more massive than an electron. It has a spin of S=0 and a mass-energy content of 939.6 MeV. Like the proton, its sub-structure has a complex configuration in (momentum) space, but like the electron, it is still comprised of a photon of the appropriate energy making two revolutions per wavelength. A neutron is neutral because it contains the charge of the proton plus the charge of the electron, though it is not neutral throughout its structure. It contains regions of positive charge and regions of negative charge. The mass of the neutron is very similar to (though slightly larger than) the combined masses of the proton (938.3 MeV) and electron (511 keV). The difference in their masses represents the difference in energy between the neutron state and the electro-proton state that is the hydrogen (H) atom. An isolated neutron is not stable and usually decays in less than 15 minutes, splitting into a proton, an electron, and emitting the additional energy (in the form of an anti-neutrino). Neutrons are necessary in any nucleus containing more than 1 proton. Protons would otherwise repel one another, but neutrons are able to bind them together electrostatically. (See The Robinson Model of Nuclear Binding. See also Robinson: The Structure of Electrons, Protons, Neutrons & Neutrinos.)
O
Orbital:
A region of space occupied by electron density. There are several different types of orbitals that vary in size and shape. The simplest is a sphere that envelops the nucleus, known as an s-orbital.
P
Paramagnetism:
This is when a substance is attracted to a magnetic field because it contains one or more unpaired electrons. In the presence of an external magnetic field, the magnetic field of an unpaired electron will cause it to align with the magnetic field. This causes cancellation of magnetic field to occur between the electron and the direction of the field, and the substance will therefore experience an attraction into the magnetic field. The dioxygen (O2) molecule and most metals are paramagnetic.
Polar:
If an atom or molecule has asymmetry in its electron cloud so that one side has more electron density than the other, it will feel partially positive on one end and partially negative on the other end. This separation of charges is called polarity, and it is analogous to the case of a magnet, which has a north magnetic pole on one end and south pole on the other. Polar molecules are attracted to one another. If the electron cloud is symmetrical or identical on opposite sides of the atom or molecule, then it will be non-polar. Non-polar molecules are not attracted to one another… until London Dispersion forces (see above) become significant.
Positron (e+):
A positively charged subatomic particle that is identical to the electron, but with a right-handed spin of S=½, a charge of +1.6×10-19 Coulombs, and a mass/energy content of 511 keV. It has a toroidal sub-structure in momentum space, and is comprised of a photon of the appropriate energy making two revolutions per wavelength. Its charge field manifests as a sphere. A positron moving backward in time is identical to an electron moving forward in time, and vice versa.
Proton (p+):
One of the three subatomic particles out of which all atoms are made. (The other two are the neutron and the electron.) A proton is positively charged and almost 2,000 times more massive than an electron. It is a fermion and has a spin of S=½, a charge of +1.6×10-19 Coulombs, and a mass-energy content of 938.3 MeV. Like the neutron, its sub-structure has a complex configuration in (momentum) space, but like the electron, it is still made up of a photon of the appropriate energy making two revolutions per wavelength. (See also Robinson: The Structure of Electrons, Protons, Neutrons & Neutrinos.) Atoms of a given element are identified according to their number of protons. This is called the atomic number, and the periodic table of the elements is laid out in order of atomic number.
Q
Quantum:
A quantum refers to the smallest amount of change or the smallest unit of something. It also implies that changes must occur in discrete steps rather than in a smooth, continuous gradient. Stairs and piano keyboards are quantized. Ramps and linear graphs are not. A “quantum” of light is called a photon, and it is the minimum amount of electromagnetic energy that can be emitted, transmitted, or absorbed. A photon can even have a very high energy, which means that each minimum packet of energy at that wavelength carries a large amount of energy. An example is the gamma ray. Electron clouds in atoms can also only manifest certain discrete electron states. This is because each electron is an identical unit, and because their interactions result in discrete, resonant, stationary wave states. Electron clouds transition between adjacent energy states by emitting or absorbing whole photons (or electrons) — one quantum at a time.
Quantum mechanics:
The science that describes light, sub-atomic particles and their quantum interactions. It is based upon the idea that all interactions are quantized. Particles and energy states can be described by equations called wave-functions, since they are resonant wave states that are made up of photons.
R
Reactivity:
This is a measure of how readily an atom will give or take electrons in order to achieve greater stability and symmetry. If it has a low ionization energy and wants to lose electrons, a high ionization energy and wants to gain electrons, or if it has a high electron affinity and thus a strong desire to gain electrons, it will react vigorously with other elements in order to make those electron exchanges.
S
Sub-quantum mechanics:
The science that extends quantum mechanics to describe the sub-structure of subatomic particles that gives rise to their properties. It encompasses the mathematics of absolute relativity (M.A.R.T.), represented by the equation, 𝒟𝜇𝚵𝒢=0, as well as quantum inversion.
V
Valence Electrons:
These are electrons in the outermost shell of the atom. Sodium (Na) has 1 valence electron. Oxygen (O) has 6. Electrons in the inner shells are known as core electrons.
BACK to top
RETURN to the Periodic Table
