
RETURN to Periodic Table
Magnesium is the 12th element on the periodic table. It has 12 protons and 12 neutrons in the nucleus, giving it a mass of 24 amu, and it has 12 electrons enveloping the nucleus.
Electron Shell
Magnesium has a di-electron making up its 3rd shell, with two full core shells within that have the identical configuration to neon. Given its larger size, the 3s2 di-electron is not as well-bound as the 2s2 di-electron on beryllium. Magnesium will therefore donate its valence electrons more readily than beryllium, but it will not be as reactive in doing so as the metals below it in Group II such as calcium, strontium, or barium.
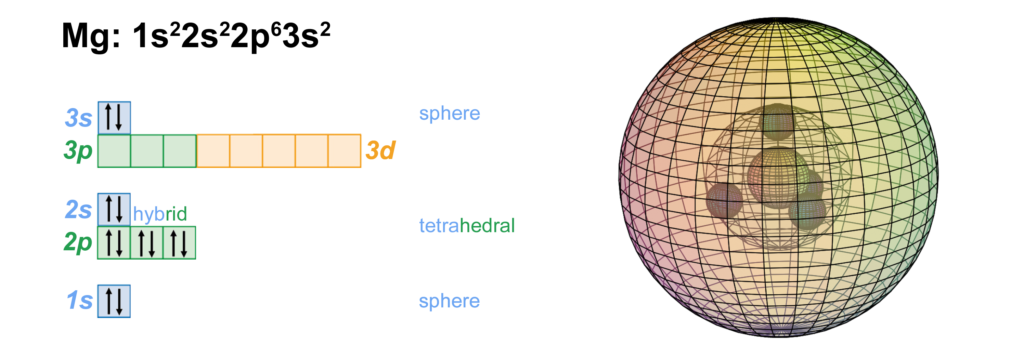
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.As we saw in the case of neon, the 2nd shell orbitals are more like spherical tetrahedra, and the 3rd shell is a di-electron in a spherical s-orbital. These orbitals represent phase-locked, resonant, coherent, harmonic, stationary waves.
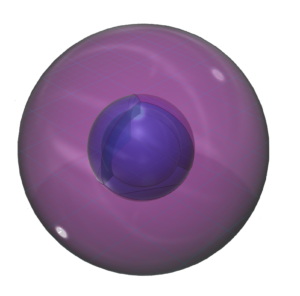
Magnesium has a smaller atomic radius than sodium. (This square represents the size of the largest atom). The decrease in size, as we move across the third row of the periodic table, results from an increase in effective nuclear charge.
Its electronegativity value makes it a metal, though slightly less metallic than sodium. This is because its stronger effective nuclear charge makes it hold its electrons more strongly.
Ion formation
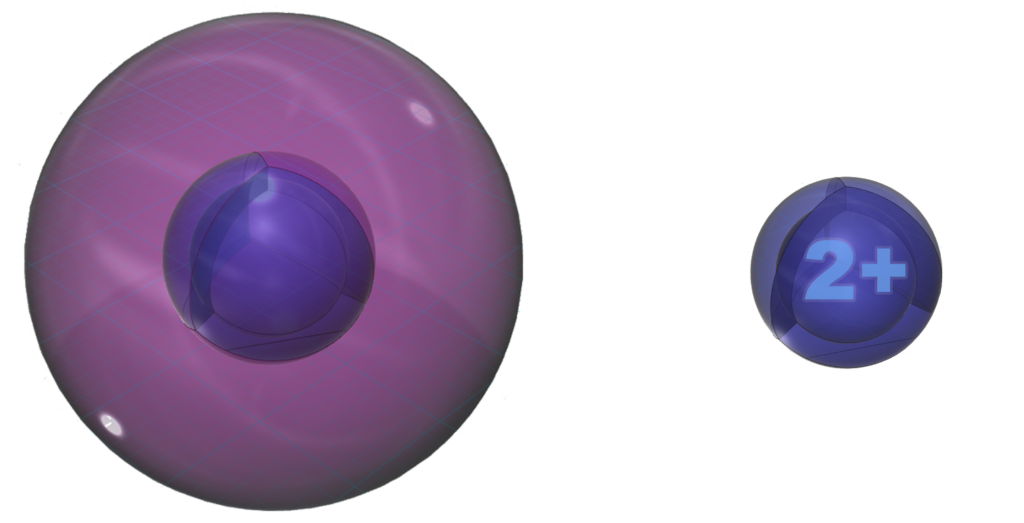
Magnesium is more willing to lose its two valence electrons in an ionic interaction than is beryllium because it is larger, and it will lose both at the same time in order to reach the stability of a full 2nd shell. This is the same electron configuration as the 2s22p6 noble gas configuration of neon — a multi-di-electron state with two concentric full shells. That is why magnesium forms a 2+ ionic state.
RETURN to the Periodic Table
OTHER GROUP II ELEMENTS: Beryllium, Magnesium, Calcium, Strontium, Barium
