
RETURN to Periodic Table
Argon is the 18th element on the periodic table. It has 18 protons and 22 neutrons in the nucleus, giving it a mass of 40 amu, and it has a full shell of 18 electrons enveloping the nucleus. Its full electron shell causes it not to react with other atoms. It is a gas under normal conditions, and it is called a ‘noble’ gas because of its non-interaction.
Electron Shell
Argon has three full electron shells. With 6 electrons in a 3p orbital, argon is believed to achieve stability with octahedral symmetry, and its 3s electrons can unhybridize and return to their preferred spherical di-electron state. It is proposed in the theory of Sub-Quantum Chemistry, however, that argon is more stable with full shells containing 4 di-electrons in sp3-hybridization (shown below).
This high degree of symmetry, with all electrons paired, renders argon, like helium and neon, unreactive.
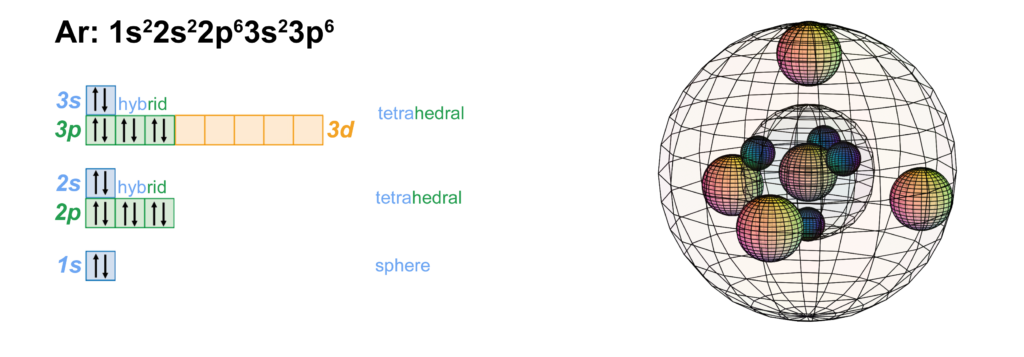
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell orbitals themselves are spherical tetrahedra, not spheres. Only s-orbitals are spherical. It is noteworthy that the tetrahedral arrangements in adjacent shells align antiparallel to each other. This places the maximum distance between electron density on adjacent shells in order to minimize electron repulsion.
As in the case of neon, if we consider the tetrahedral full-shell configuration, then argon will have a nested spherical tetrahedral orbital arrangement, the 2nd and 3rd shells each containing four di-electrons (below, right).
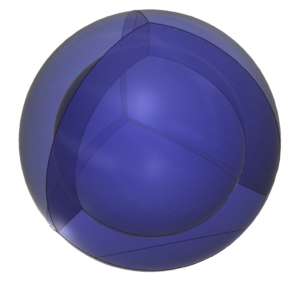

Intuitively, this seems more stable as it involves a greater degree of field cancellation than a full p-orbital with a single electron occupying each lobe. The entire shell contains electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals, where electron density will be lowest (though not zero). Together, all atomic orbitals constitute a single, phase-locked, resonant, coherent, harmonic, stationary wave.
As mentioned above, the nested tetrahedral shells align in an opposite or antiparallel fashion. The reason this geometry is so stable for electrons is that the region of lowest electron density on one shell lines up with the region of maximum electron density on the adjacent shell, minimizing repulsion. In the diagram (below), the vertex represents where the nodes between orbitals intersect. This is a point of lowest electron density. It lies directly over the center of the face of the orbital beneath it, which is the point of highest electron density in that orbital. This is therefore the lowest energy state that nested tetrahedral shells can achieve.
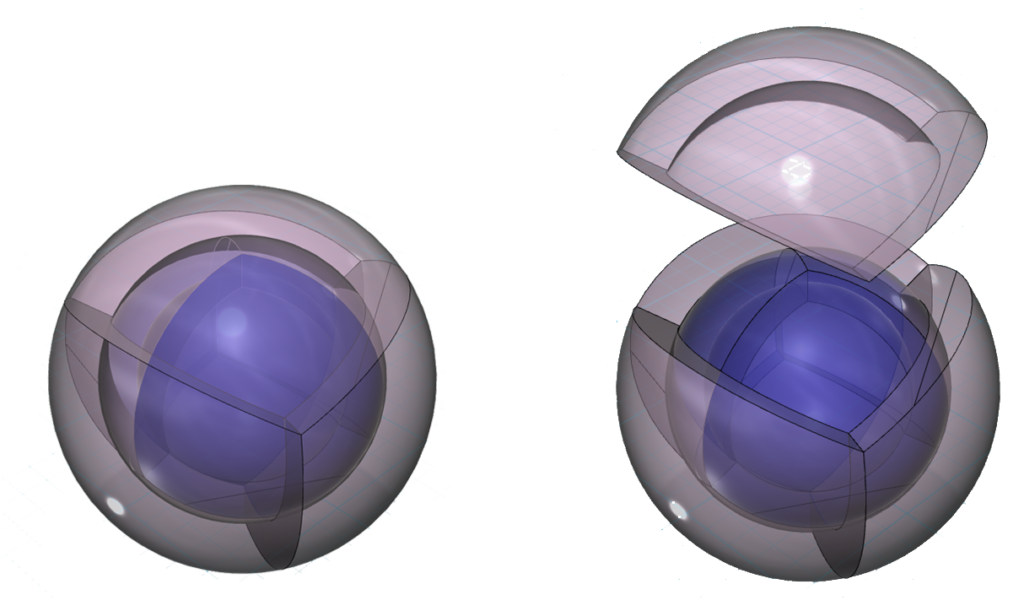
RETURN to the Periodic Table or COMPARE to Neon.
SEE OTHER NOBLE GASES: Helium, Neon, Argon, Krypton, Xenon
