
RETURN to Periodic Table
The elements of Group VIII have full electron shells with all electrons paired. They are called the Noble Gases because they are generally stable and do not react or bond with other atoms. There are exceptions to this, however, as we move down the group to the larger atoms.
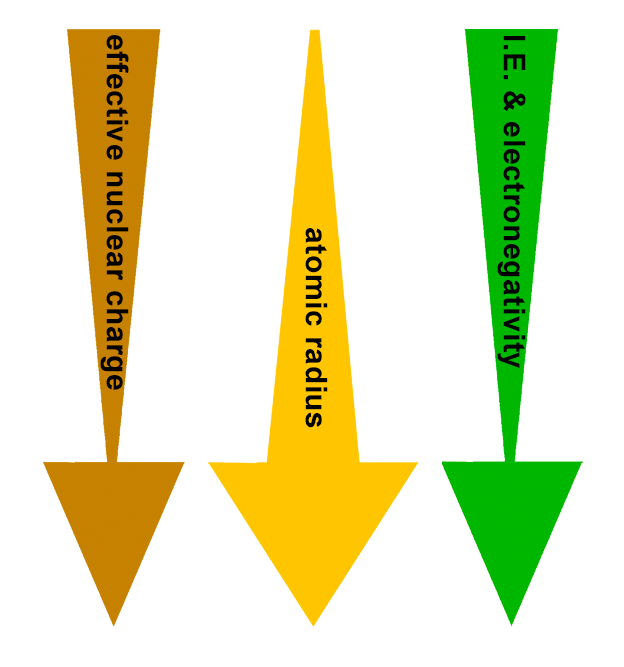
PERIODIC TRENDS:
Effective nuclear charge (Zeff) decreases down the group.
Therefore:
Atomic radius increases down the group.
Ionization energy decreases down the group.

The ionization energy of each atom in the 8th group is the highest in its row, but as we move down the group, atomic radius increases as new shells are added. This means that valence electrons are further from the attraction of the nucleus, and therefore more susceptible to bonding. While the smaller noble gases do not have electronegativity values because they do not bond, the larger ones can. This is because ionization energy, while high in this group, decreases down the group, rendering the atoms more able to react.

HELIUM (2He)
Helium has a full 1st shell made up of a single di-electron. It is the smallest and most stable atom, and therefore has the highest ionization energy on the periodic table (2,372 kJ/mol), almost double that of hydrogen (1,312 kJ/mol). As such, helium does not bond with any other atom because that would entail either sharing or losing an electron.

NEON (10Ne)
Neon has a full 2nd shell made up of four di-electrons in tetrahedral symmetry. It has a high ionization energy (2,081 kJ/mol), but not as high as helium because it is a larger atom, with valence electrons further from the attraction of the nucleus. But its ionization energy is still too high to allow bonding. As such, neon does not bond with any other atom because that would entail either sharing or losing an electron.
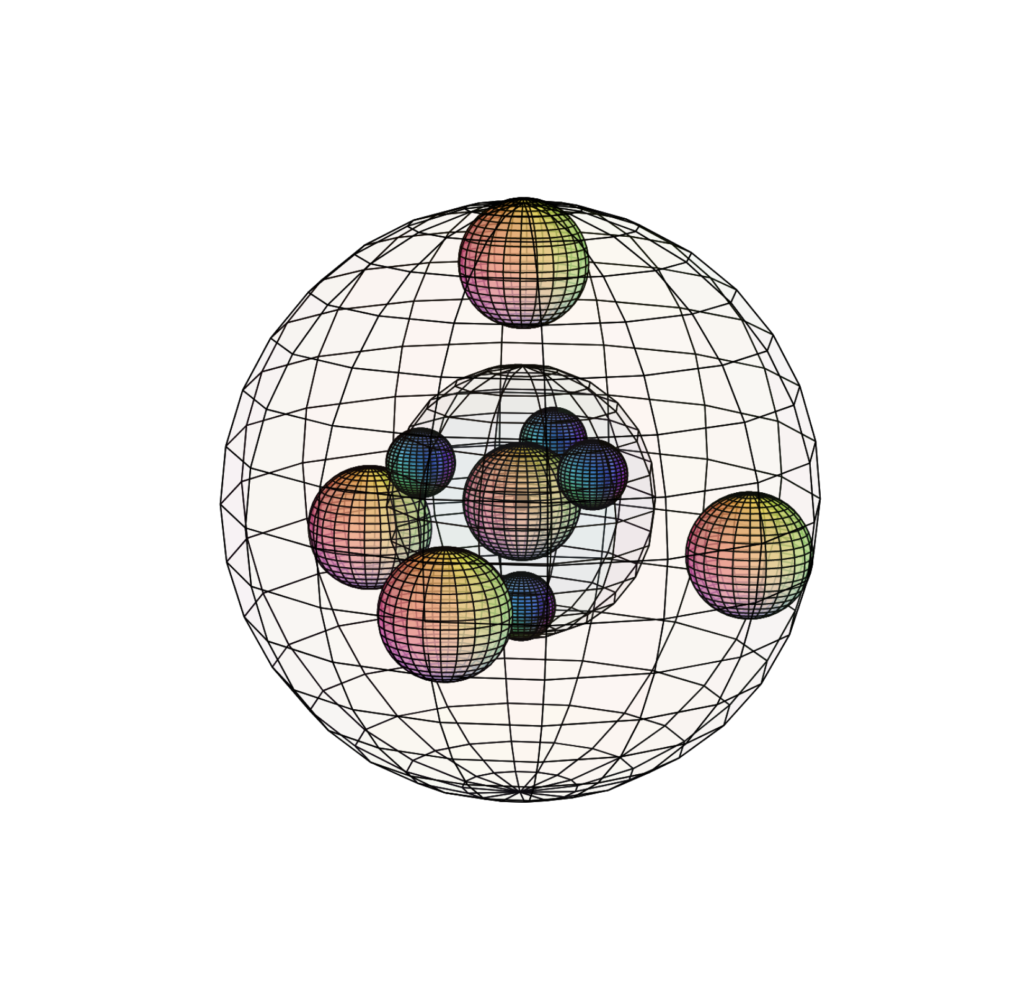
ARGON (18Ar)
Argon has a full 3rd shell made up of four di-electrons in tetrahedral symmetry. As a larger atom with an added electron shell, argon has a lower ionization energy (1,521 kJ/mol) than neon, and in fact, lower even than that of fluorine (1,681 kJ/mol). This means that under the right conditions, fluorine can be forced to bond with argon, as in the molecule HArF.

KRYPTON (36Kr)
Krypton has a full 4th shell made up of four di-electrons in tetrahedral symmetry. As a larger atom, krypton has a lower ionization energy (1,351 kJ/mol) than argon, lower even than that of fluorine (1,681 kJ/mol), and almost as low as oxygen (1,314 kJ/mol). This means that under the right conditions, fluorine can be forced to bond with krypton, as in the molecule KrF2.

XENON (54Xe)
Xenon has a full 5th shell made up of four di-electrons in tetrahedral symmetry. As a larger atom, xenon has a lower ionization energy (1,170 kJ/mol) than krypton, lower even than that of fluorine (1,681 kJ/mol) and oxygen (1,314 kJ/mol). This means that under the right conditions, fluorine and oxygen can be made to bond with xenon. Examples include the molecules XeO2, XeF4, and XeF6.
RADON (86Rn) is not shown.
PERIODIC TRENDS: PERIOD II, PERIOD III, GROUP I, GROUP II, GROUP VII, GROUP VIII
RETURN to the Periodic Table
