
RETURN to Periodic Table
Below: Electron Shell, Bonding & Ion Formation, Magnetic Properties
Scandium is the 21st element on the periodic table. It has 21 protons and 24 neutrons for a mass of 45 amu, and 21 electrons.
Electron Shell 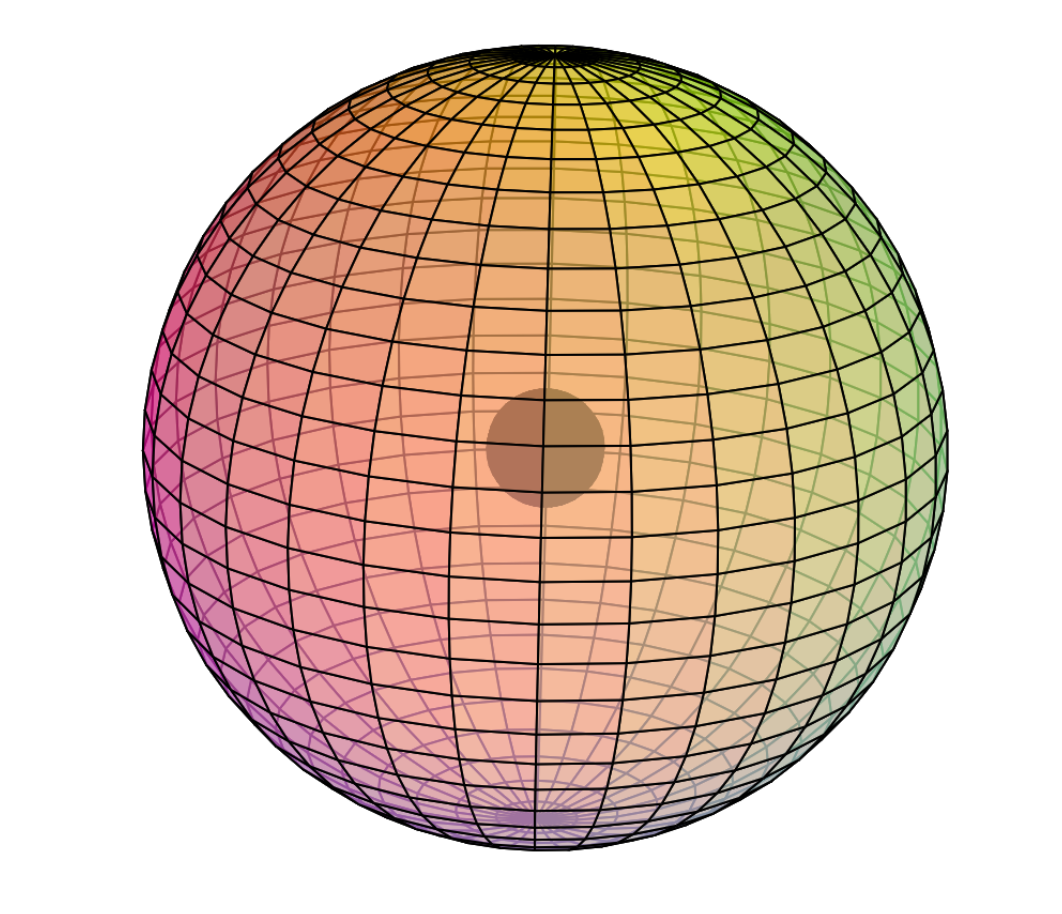
DISCLAIMER: The following reflects the sub-quantum mechanics approach to electron interactions and hybridization. Some details may therefore differ somewhat from traditional quantum chemistry:
Scandium is the first element to feature electrons in the d–orbital. Since d-electrons are resonating in the same space as p-electrons within their parent s-shell, we propose that these orbitals are able to hybridize together in search of lowest energy and highest stability. [Ref] If that is the case, scandium would feature a 3rd shell containing 3 di-electrons and 1 unpaired electron in p3d-hybridized tetrahedral symmetry. This pd-hybridization does not need to involve the 3s-orbital electrons, and they can return to their preferred spherical di-electron state. (see image below)
The 3rd shell tetrahedral symmetry will also align antiparallel (and antiprismatic) to the 2nd shell tetrahedral electron arrangement within, in order to minimize di-electron repulsion between shells.

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectNOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell hybrid orbitals themselves will assume a spherical tetrahedral structure that divides the shell into four roughly equal volumes. Each shell segment will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals — as wave structures usually do — where electron density will be lowest (though not zero).
NOTE ALSO: Even though it is often useful to talk about these orbitals as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in this harmonic wave structure.
An alternative representation of the scandium atom is given below. It shows a 3rd shell p3d-hybridised tetrahedral symmetry that is similar to the electron configuration of chlorine — 3 di-electrons and 1 unpaired electron.
A key difference, though, is that these hybrid orbitals exist within the added sheath of the 3s2-orbital di-electron, since it is not needed for hybridization. It is proposed that the 3s2 di-electron gives additional stability to the tetrahedral p3d-hybrid orbitals superimposed within it — like harmonic frequencies upon a fundamental — even though it contains an unpaired electron.

The remaining elements in the d-block will also experience this 3s-orbital stabilization phenomenon, since they also achieve at least 4-directional symmetry with only their p– and d-orbitals.
Bonding & Ion Formation 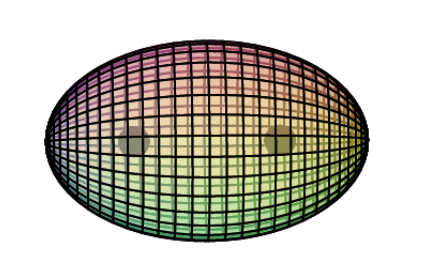
The 4s2-orbital di-electron shell completes the atom. When forming a metallic crystal, the 4s2 electrons delocalize to form the metallic bond, and when forming a 2+ ion, they are removed. In both cases, the core electron geometry remains the same. (A third ionization, however, would change it to an sp3-hybridized tetrahedral structure.)
(In a few cases, such as chromium (Cr) and copper (Cu), the 4s-orbital outer shell contains only 1 electron, since the other joins the 3rd shell hybridization within in order to help it achieve 8-directional symmetry.)
Magnetic Properties 
Scandium has one unpaired electron. In all previous elements, unpaired electrons have occurred in the outer (valence) shell of the atom. Those are the electrons involved in chemical reactions. Scandium is the first case of an atom with an unpaired core electron. It is protected from reacting (at least, until scandium becomes an Sc2+ ion), and it is stabilized by the 3s di-electron ‘fundamental.’ We propose that it is this protection and stabilization of unpaired core electrons that allows them to interact magnetically, and that consequently determines an element’s magnetic properties.
UNPAIRED CORE ELECTRONS:
While these electrons may technically be called core electrons, for the purposes of this discussion, we recognize that in a metal, if we exclude the valence “conduction” electrons, the pd-hybrid orbital electrons do become ‘valence electrons’ in a sense. Not in the sense that they can participate in chemical reactions, but in the sense that they are now in the outermost shell of the atomic cores, which are suspended in the conduction electron matrix — the 3D electron gas — of the solid metal crystal.
PARAMAGNETISM:
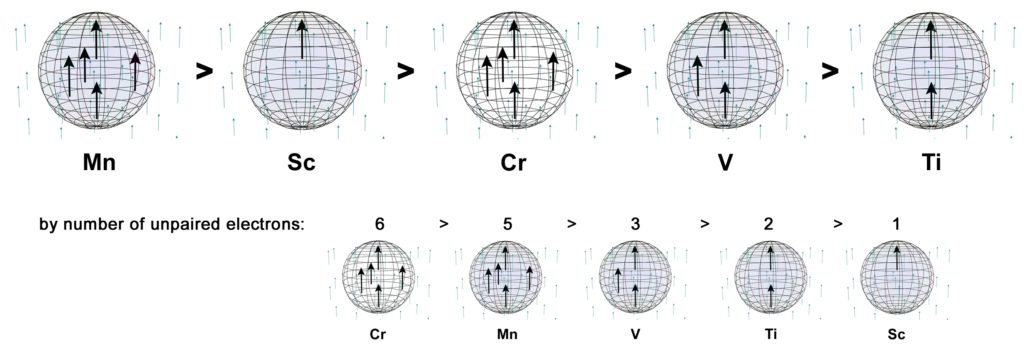
In the presence of an external magnetic field, an unpaired core electron will orient its spin to align with the magnetic field. This will cause the atom to be drawn into and toward that field — via magnetic field cancellation — giving it a positive magnetic susceptibility (χm) value. This attractive force is called paramagnetism, and its effects only last as long as the external magnetic field is present. We might therefore presume that, the stronger the paramagnetism, the more ‘unpaired electron character’ is present. Surprisingly, this does not seem to go according the number of unpaired electrons present, as the diagram below illustrates. There must therefore be other contributing factors, which we will investigate below.
Despite only have a single unpaired electron, scandium is the second most paramagnetic metal of the 3d row — excluding the ferromagnetic iron, cobalt, and nickel.
As proposed above, scandium has one unpaired electron in one position of a tetrahedral electron structure. The images below are intended to represent the orientation of this 3rd shell electron in an external (or adjacent atom’s) magnetic field (whose north pole is pointing upwards). (The purple arrow inside the atom represent the unpaired electron and the black dots represent the relative positions of the 3 di-electrons in that shell.)

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectIt is here proposed that scandium represents a rather simple case of one electron that is susceptible to an external magnetic field, and whose effects are not mitigated by other unpaired electrons within the core electron topology. As we will see in the case of some other d-block metals, the presence of unpaired electrons spin-bonding within an atomic shell can cancel magnetic field and reduce an electron’s effective magnetic signature with respect to an external magnetic field. Without such mitigation, scandium’s one unpaired electron will have what we might call an ‘Effective Magnetic Electron’ count of EME ≈ 1e–, yielding a strong magnetic susceptibility value of χm = +295.
PARAMAGNETIC STRENGTH ANALYSIS:
The following diagram shows the relative paramagnetic strengths of the transition metals, along with their proposed hybrid orbital geometries. (See paramagnetic strength trend analysis for more detail.)
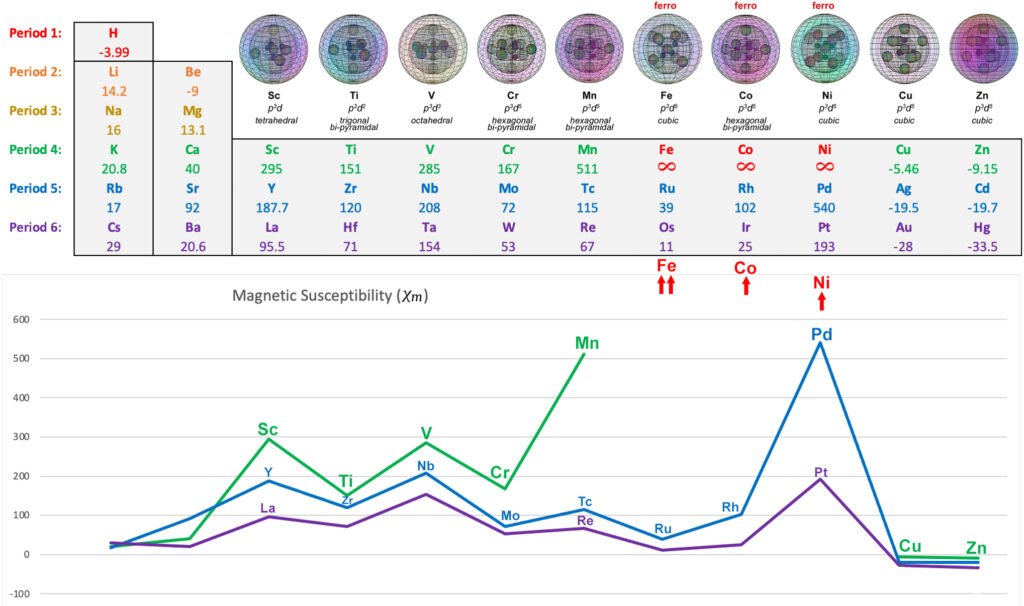
OTHER PARAMAGNETIC 3d METALS: Scandium, Titanium, Vanadium, Chromium (also antiferromagnetic), Manganese
FERROMAGNETIC 3d METALS: Iron, Cobalt, Nickel
DIAMAGNETIC 3d METALS: Copper, Zinc
RETURN to the Periodic Table
