
RETURN to Periodic Table
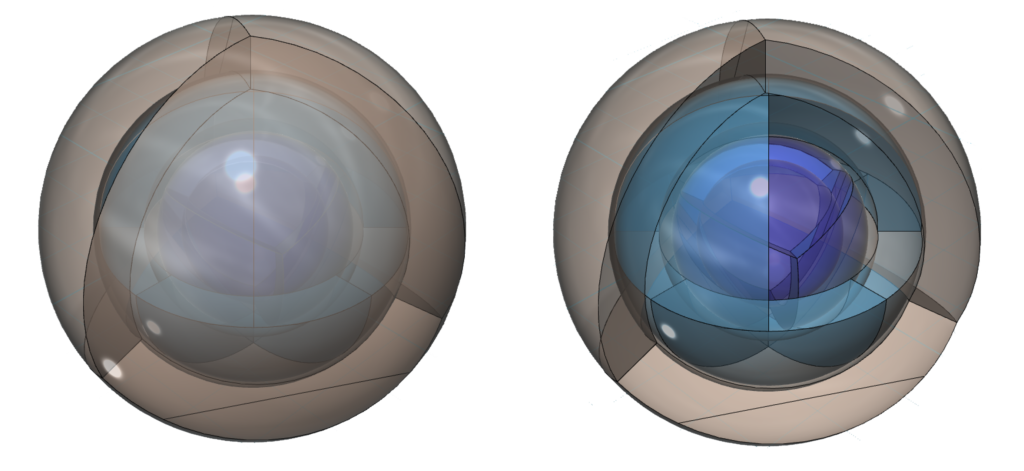
Krypton is the sixth element with electrons in the 4p orbital. Krypton has four full shells. While the smaller noble gases are unreactive, krypton has a lower ionization energy due to its larger size to the point that it can bond with the very electronegative fluorine atom. (The wireframe indicates the boundary of the n=4 shell.)

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectAn alternate view (shown below) of the orbitals of krypton shows an innermost 1st-shell sphere within a 2nd tetrahedral shell (dark blue) within a 3rd cubic anti-prismatic shell (light blue) within an anti-aligned 4th tetrahedral shell (brown).
RETURN to the Periodic Table
SEE OTHER NOBLE GASES: Helium, Neon, Argon, Krypton, Xenon
