
RETURN to Periodic Table
Nitrogen is the 7th element on the periodic table because it has 7 protons in its nucleus. The protons will attract 7 electrons to surround the nucleus in order to form a neutral atom. With 7 protons and 7 neutrons, most nitrogen atoms have an atomic mass of 14 amu.
Electron Shell
Nitrogen has three electrons in its p-orbital. This is not a sphere-shaped harmonic, and so three p-electrons cannot achieve a stable electron symmetry around a spherical core. Nitrogen therefore cannot simply add its three p-electrons on top the same (2s2) configuration that beryllium has, as shown here, because it would not be a stable configuration:
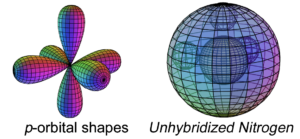
The asymmetry therefore causes the three p-orbital electrons to combine (hybridize) with the two s-orbital electrons in order to achieve 4-directional, symmetry. Nitrogen’s tetrahedral (sp3) symmetry features one di-electron (lone pair) and 3 unpaired, degenerate electrons. This is why nitrogen typically makes 3 bonds. The quantum states of the three degenerate electrons become linked, which stabilizes them and thereby increases nitrogen’s ionization energy. (The large wireframe below indicates the boundary of the n=2 shell.)

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object. NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 2nd shell hybrid orbitals themselves will be more like spherical tetrahedra that fill the volume within their shell with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals — as wave structures usually do — where electron density will be lowest (though not zero).
NOTE ALSO: Even though it is often useful to talk about these orbitals as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in this harmonic wave structure.
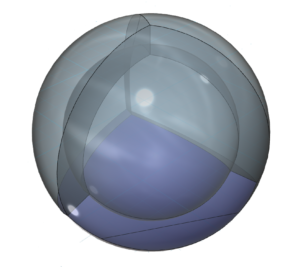
In the case of nitrogen, the orbital containing the ‘di-electron’ lone pair (dark blue below) will occupy slightly more volume than the three containing an unpaired electron.


Nitrogen has a smaller atomic radius than carbon. (This square represents the size of the largest atom).
Both its decrease in size and its increase in electronegativity (across the period) result from its larger effective nuclear charge. Its resulting unwillingness to give up an electron makes it strongly non-metallic.
Bonding & ion formation
Nitrogen is keen to obtain three extra electrons to fill its 2nd shell and achieve maximum stability. That would be the same electron configuration as the 2s22p6 noble gas configuration of neon — a multi-di-electron state with two concentric full shells.
One way nitrogen can do this is by making three covalent bonds. An important example of this is the triple bond that nitrogen atoms make with each other when forming the nitrogen molecule (N2).

A triple bond forms when the three unpaired electrons on one nitrogen atom each pair with one of the unpaired electrons on the other nitrogen atom. (This image is not meant as an accurate depiction of the bond formation.) In the Lewis Dot structure below it, the two pairs of black dots represent the remaining di-electrons on each atom.
A triple bond is a strong bond, and this makes it more difficult for nitrogen molecules to be broken apart. This causes more of them to persist, and this is the reason the Earth’s atmosphere contains mostly nitrogen molecules (78%). Oxygen molecules are bound with a weaker double bond, and they are therefore more reactive, more easily separated, and therefore a smaller portion of them (21%) remain in the atmosphere.
This is also why plants need the help of soil bacteria, who use their biological enzymes to aid in the conversion of atmospheric nitrogen gas (N2) into a more usable organic form, such as nitrate (NO3–) or nitrite (NO2–). The plants are then able to absorb these ions through their roots and use them.
Nitrogen can also fill its 2nd shell by gaining three electrons in ionic interaction. That is why nitrogen forms a 3– ionic state. The negative ion is much larger than the neutral atom because electrons now outnumber protons by three. This results in a much lower effective nuclear charge — a lower average attraction by the nucleus on each electron, which expands the size of the electron shell as it is attracted inward with less force.

Ammonia (NH3)
When nitrogen bonds covalently with three hydrogen atoms, the biologically important ammonia (NH3) molecule is formed. Like the water molecule, its asymmetrical structure gives it important properties. The most significant is that it creates an electron imbalance which makes the side of the molecule where the hydrogen atoms attach slightly positive (𝛿+) and the opposite (nitrogen) side slightly negative (𝛿–). The presence of the di-electron (lone pair) on the nitrogen atom also makes this molecule alkaline (basic) in solution.
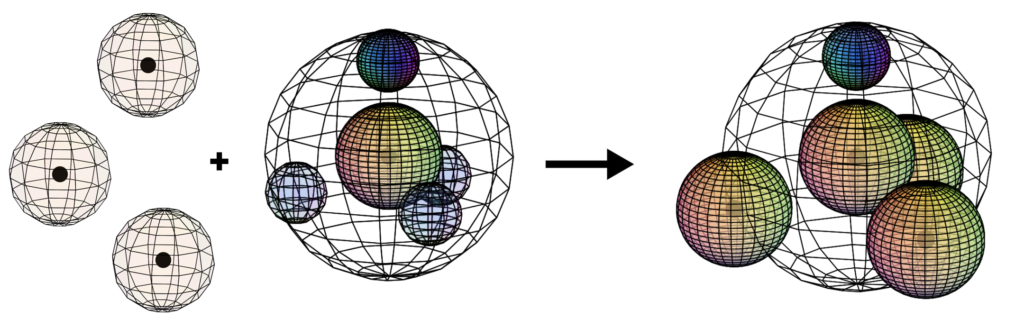
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectThe ammonia molecule is vitally important in the manufacture of fertilizer. The invention of a process to make it efficiently (by Fritz Haber) revolutionized humanity’s ability to provide enough agricultural produce to feed our growing global population.
RETURN to the Periodic Table
