
RETURN to Periodic Table
Fluorine is the 9th element on the periodic table because it has 9 protons in its nucleus. The protons will attract 9 electrons to surround the nucleus in order to form a neutral atom. With 9 protons and 10 neutrons, most fluorine atoms have an atomic mass of 19 amu.
Electron Shell
Fluorine has five electrons in its p-orbital. This is not a sphere-shaped harmonic, and so five p-electrons cannot achieve a stable electron symmetry around a spherical core. Fluorine therefore cannot simply add its five p-electrons on top of the same (2s2) configuration that beryllium has, as shown here, because it would not be a stable configuration:
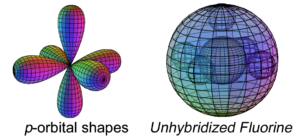
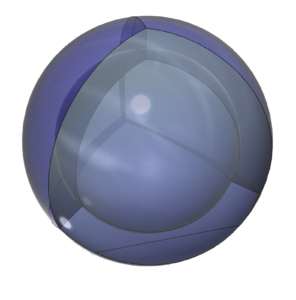
The asymmetry therefore causes fluorine to hybridize its 2s and 2p electrons in order to achieve tetrahedral symmetry. Its sp3 hybrid orbitals feature three di-electrons and one unpaired electron, rendering it extremely (and dangerously) reactive in search of that final electron-pairing. One more electron will give it a full 2nd shell, like neon, and that is a very attractive state for the atom. In addition, a high effective nuclear charge gives fluorine the highest electronegativity in its row, and because it is the smallest of the Group VII elements, its electronegativity is also the highest on the periodic table. (The large wireframe below indicates the boundary of the n=2 shell.)

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 2nd shell hybrid orbitals themselves will be more like spherical tetrahedra that fill the volume within their shell with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals — as wave structures usually do — where electron density will be lowest (though not zero).
NOTE ALSO: Even though it is often useful to talk about these orbitals as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in this harmonic wave structure.
In the case of fluorine, the three orbitals containing di-electrons will each occupy slightly more volume than the one containing the unpaired electron. This will cause the unpaired electron orbital to be slightly constricted, which may also cause it to become slightly extended above an otherwise spherical surface of the electron cloud. It is conceivable that this may further exacerbate fluorine’s high reactivity. [Ref]


Fluorine has a smaller atomic radius than oxygen. (This square represents the size of the largest atom).
Both its small size and the fact that it has the highest electronegativity (on the period table) result from its very high effective nuclear charge, making is extremely non-metallic and extremely reactive.
Bonding & ion formation
Fluorine is so eager to obtain an extra electron to fill its second shell that it can bond with just about any atom on the periodic table, even the larger (and usually-unreactive) noble gases, forcing them to donate electrons into that bond. Fluorine can therefore make a single covalent bond, achieving the same electron configuration as the 2s22p6 noble gas configuration of neon — a multi-di-electron state with two concentric full shells.
Fluorine can also gain an electron in an ionic interaction in order to reach the stability of a full 2nd shell. That is why fluorine forms a 1– ionic state. The negative ion is larger than the neutral atom because electrons now outnumber protons by one. This results in a lower effective nuclear charge — a lower average attraction by the nucleus on each electron.

Some properties & uses
With the highest electronegativity, fluorine is the most reactive element on the periodic table. Fluorine gas (F2) is so reactive that if it is simply passed over carbon, the carbon will spontaneously combust in it. In contrast, if we pass oxygen gas over carbon, it will only combust if ignited.
Although hydrofluoric acid (HF) is a weak acid, it cannot be stored in a glass container because it will degrade the glass due to fluorine’s high reactivity.
RETURN to the Periodic Table
OTHER GROUP VII HALOGENS: Fluorine, Chlorine, Bromine, Iodine
