
RETURN to Periodic Table
Below: Electron Shell, Bonding & Ion Formation, Magnetic Properties, Temperature
Cobalt is the 27th element on the periodic table. It has 27 protons and 32 neutrons for a mass of 59 amu, and 27 electrons.
Electron Shell 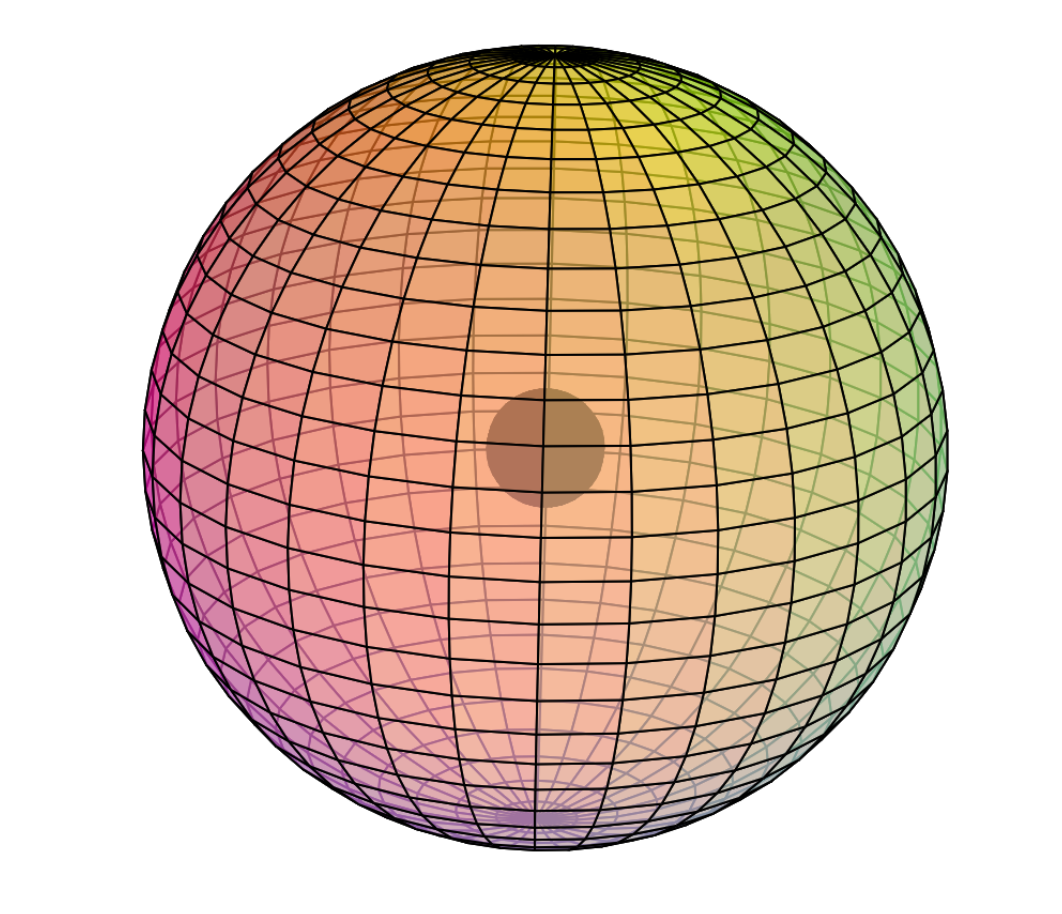
DISCLAIMER: The following reflects the sub-quantum mechanics approach to electron interactions and hybridization. Some details may therefore differ somewhat from traditional quantum chemistry:
Cobalt is the seventh element with electrons in the d–orbital. Building upon the pd-hybridization [ref] we introduced in regard to the previous d-block transition metals, it is proposed that cobalt has a 3rd shell containing 5 di-electrons and 3 unpaired electrons in p3d5-hybridized, hexagonal bipyramidal symmetry. The 5 di-electrons occupy three symmetrically distant equatorial positions and the two axial positions, in order to minimize repulsion. The 3 unpaired electrons occupy the remaining equatorial positions in between. (This is a similar electron structure to manganese, which has 3 di-electrons and 5 single electrons in this same configuration.)
In such a case, the 4 tetrahedral 2nd shell di-electrons will align themselves with one di-electron opposite one of the single electrons in the 3rd shell, allowing all other 2nd and 3rd shell di-electrons to orient their directions roughly between one another in order to minimize repulsion between shells.
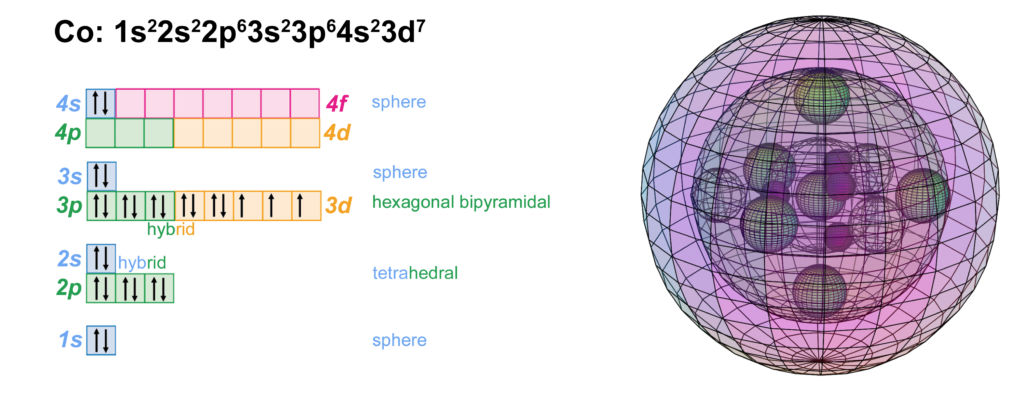
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectNOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 3rd shell hybrid orbitals themselves will assume a spherical hexagonal bipyramidal structure that divides the shell into eight roughly equal volumes, with a 5-way and a 3-way symmetry. The entire shell will be filled with electron density. It will be highest at the center of the face of each orbital (as in the traditional hybrid orbital lobe shapes) and will decrease toward the nodal regions between orbitals — as wave structures usually do — where electron density will be lowest (though not zero).
NOTE ALSO: Even though it is often useful to talk about these orbitals as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in this harmonic wave structure.
The diagram below shows only cobalt’s eight 3rd shell p3d5-hybrid orbitals. (The darker blue color represents di-electron orbitals, the lighter blue color represents unpaired electron orbitals.) In reality, the eight volumes will not be exactly equal in size because di-electron orbitals (with charge 2-) will repel the unpaired electron orbitals (with charge 1-) more strongly, constricting them. It is therefore proposed that cobalt’s unpaired electron orbitals will form an equatorial, trigonal planar geometry with respect to one another, and that they will become somewhat extended, radially outward, as a result of this constriction. (The axial and equatorial di-electron orbitals will also differ somewhat in size due to their different 2-way versus 3-way geometric constraints.)
Bonding & Ion Formation 
When cobalt atoms bonds with other metal atoms in a solid, they form a crystal structure in which their valence electrons become ‘delocalized’ — shared into a matrix of electron density within which the now-positive atomic cores remain suspended. They are held in their relative positions by a balance between attraction into the electron gas around them and repulsion from the adjacent positive atomic cores. This is the electrostatic nature of metallic bonding. Its electron delocalization is also the reason that metals are such good conductors of electrical potential.
When cobalt interacts ionically, it usually makes the Co2+ or the Co3+ ion (though there are other possibilities). The Co2+ ion forms when the atom loses its 4s2 valence electrons. The Co3+ ion forms when the Co2+ ion loses another electron. It is here proposed that the 3rd electron will be lost from one of the di-electrons, since this is the only way to retain 8-directional symmetry. The Co3+ ion will therefore achieve the same cubic (double-tetrahedral) electron configuration we saw in iron (Fe), though without any 4s-electron density.
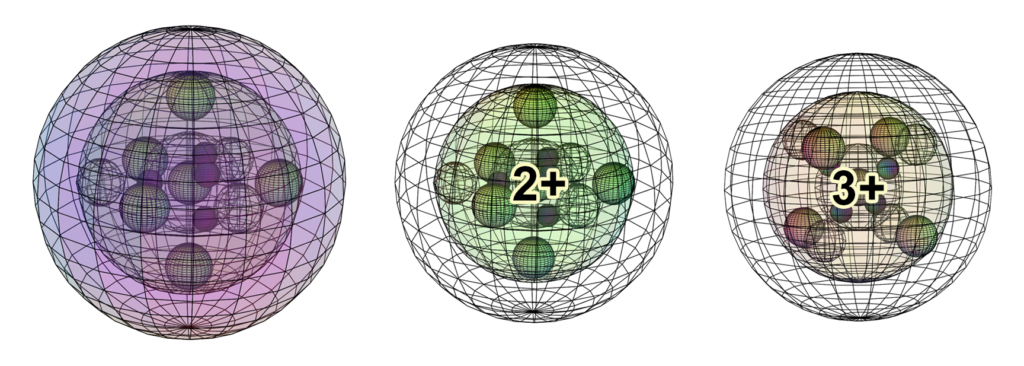
In ionic crystals — without delocalized electron density — cobalt atoms no longer conduct electricity. They will, however, still be magnetically active because they still hold unpaired electrons. While metallic cobalt is ferromagnetic (see below), cobalt ions will be strongly paramagnetic.
Magnetic Properties 
Iron (Fe), cobalt (Co), and nickel (Ni) are ferromagnetic. Cobalt is much less ferromagnetic than iron, but more so than nickel. (See Magnetism for more detail.)
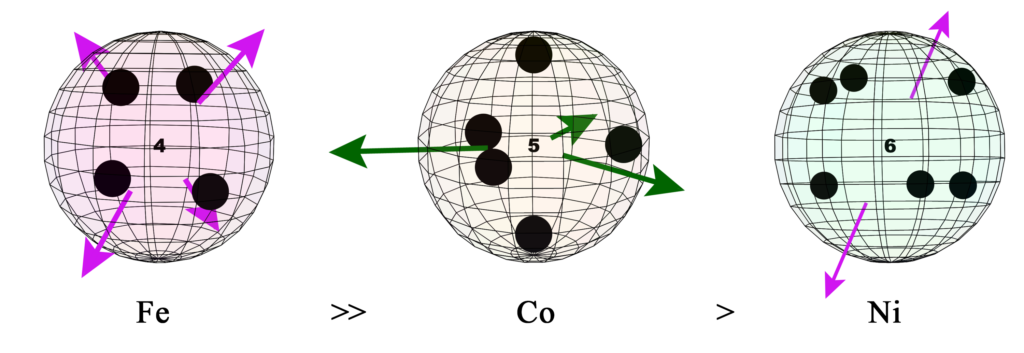
UNPAIRED CORE ELECTRONS:
The presence of unpaired core electrons determines an element’s magnetic properties. While these electrons may technically be called core electrons, for the purposes of this discussion, we recognize that in a metal, if we exclude the valence “conduction” electrons, the pd-hybrid orbital electrons do become ‘valence electrons’ in a sense. Not in the sense that they can participate in chemical reactions, but in the sense that they are now in the outermost shell of the atomic cores, which are suspended in the conduction electron matrix — the 3D electron gas — of the solid metal crystal.
Unpaired core electrons are protected from reacting with other atoms. They are held in their geometry by atomic orbital constraints, they are kept from pairing up with each other by the spin and field exclusion of degeneracy, and it is proposed that they are stabilized by the presence of the 3s2 orbital ‘fundamental’ [ref].
PARAMAGNETISM:
Protected unpaired core electrons are, however, still free to respond to and orient themselves with an external magnetic field. In doing so, they cancel magnetic field with that external field (through destructive interference), which lowers energy and attracts them towards the field. The paramagnetic atom as a whole is then attracted, along with these electrons, towards the magnetic field — attracted equally to the ‘north’ as to the ‘south’ polarity. This is called paramagnetism, and it is an expected property of metals with unpaired core electrons.
When the external field is removed, the electron spins within adjacent paramagnetic atoms return to their previous, random distribution. (See paramagnetic strength trend analysis for more details.)
FERROMAGNETISM:
As we discussed regarding iron (Fe), however, ferromagnetism occurs when the electrons in a substance are able to retain their internal crystalline magnetic field alignment after the external field that aligned them is removed. The solid can then act as a permanent magnet. This property therefore depends directly upon how well the unpaired electrons on adjacent atoms can link their spins and magnetic fields, thereby holding one another in alignment.
It is here proposed that there are two criteria that are required in order for the atoms of a metal crystal to achieve ferromagnetic spin bonding, and thus, a crystal-wide ferromagnetic spin resonance.
- CRYSTAL GEOMETRY: For optimal spin bonding, unpaired core electrons should have an electron domain geometry that matches the crystal unit cell geometry. We propose that the 8-directional symmetries that arise as a result of pd-hybridization may provide these.
- SUFFICIENT ORBITAL EXTENSION: Unpaired core electrons require constriction and radial extension in order to interact (via spin bonding) with similarly extended core electrons on adjacent atomic cores in a crystal. We propose that this is achieved as a result of sufficient di-electron (electron pair) repulsion within the atomic shell, along with added repulsion from the di-electrons in the 2nd shell that lie directly beneath the unpaired core electron orbitals. This criterion is therefore intimately related to the electron geometry (from criterion #1 above), as well as to the number of same-shell di-electrons constricting the unpaired electron orbitals.
Only with both of these criteria fulfilled (see below), will a transition metal be ferromagnetic. If only one is present, or if constriction and extension are not sufficient, the element will be paramagnetic.
For more on the relative trend in ferromagnetic strength, see Magnetism & Magnetic Trends.
CRITERION #1: COBALT’S CRYSTAL GEOMETRY:
Cobalt is ferromagnetic below its Curie Temperature (of 1,115°C) — which is much higher than iron’s Curie Temperature. It has a hexagonal close-packed (HCP) crystal structure below about 450ºC, and a face-centered (FCC) crystal structure above 450ºC. When heated past its Curie Temperature, cobalt loses its ferromagnetism in favor of paramagnetism.
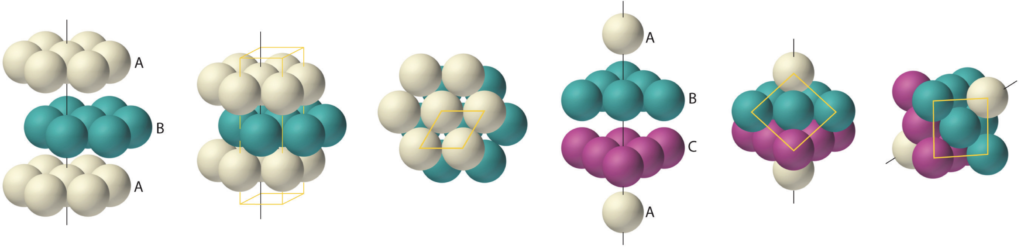
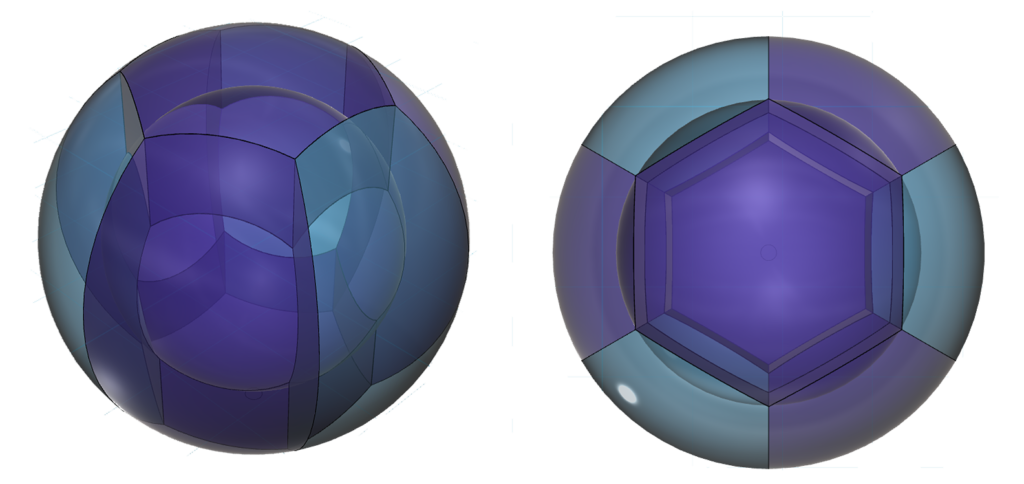
In a normal hexagonal close-packed (HCP) structure (above, left), hexagonal planar layers stack on top of one another, with the atoms of one layer nestling in between the atoms of the adjacent layer for maximum packing density. There are two possible ways to offset the second layer, so there are two variations on an A-layer’s position — called a B- or a C-layer. In HCP, the layers alternate between the A and B alignments only, resulting in a crystal in which the atoms of every other layer are vertically aligned in an A-B-A-B-etc pattern. (As we see in the top view in figure 5, above, this leaves some thin columnar gaps through the crystal.)
In a face-centered cubic (FCC) crystal (fig. 5 above, on the right), the layers cycle through both offset positions, creating an A-B-C-A-etc pattern. The difference between the B- and C-layers is a rotation through 30º of the hexagonal layer. In figure 6 (below, right), that would be the equivalent of having the three purple spheres nestled in the other three of the six positions between the yellow spheres. This structure is also known as cubic close-packing (CCP).
As we see in the ‘cubic’ view (in fig. 5, above, far right), unlike HCP, the FCC structure leaves no columnar gaps through the crystal. (This can be more clearly seen in figure 7 below.) In both close-packing structures, HCP and FCC, each atom has 12 nearest neighbors.
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.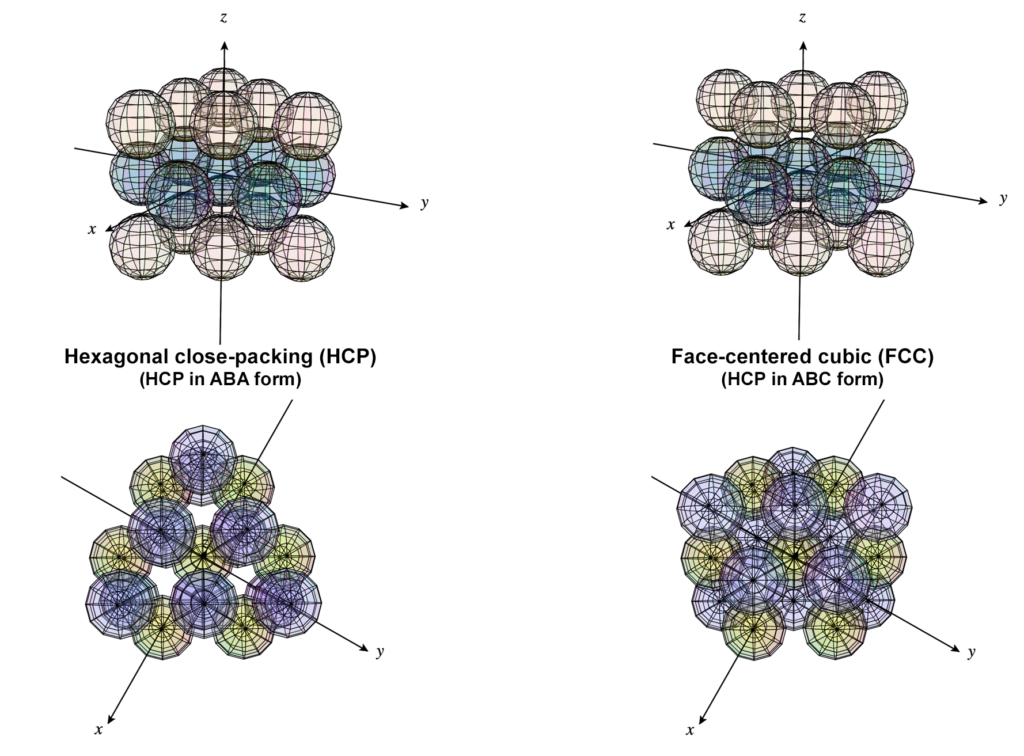
 CLICK HERE to interact with this object; and face-centered cubic (FCC) on the right —
CLICK HERE to interact with this object; and face-centered cubic (FCC) on the right —  CLICK HERE to interact with this object.
CLICK HERE to interact with this object.According to cobalt’s proposed electron domain geometry, its 3 unpaired electron orbitals, in an equatorial trigonal planar arrangement, would align perfectly within the hexagonal layers of both HCP and FCC crystal geometry. Every other corner of each ‘hexagon’ would then find three extended unpaired electron orbitals, one from each of three adjacent atoms, attracted into a coherent ‘tri-electron’ Parallel Spin Bonding (PSB) state in the electron gas ‘gaps’ between the atoms in the crystal. (These are the groups of 3 pink dots in fig. 6 above).
The other three gaps will therefore find three di-electrons approaching each other, one from each atom. (These are the groups of 3 black dots in fig. 6). As in the case of iron, these di-electron orbitals repel each other. Since they are neither constricted nor extended, they will not be as close to one another, as will the spin bonding unpaired electrons (and they may perhaps behave almost like ‘diamagnetic bumpers,’ adding stability to the coherence of the crystal state.)
Within each layer in the crystal, the spin-bonding and diamagnetic relationships between the atoms will be the same, whether it is in HCP or FCC form. While this seems to be essentially a planar resonance through the crystal, as we will see, it is proposed that the relative rotation of the layers — HCP versus FCC — can also allow or prevent an additional crystalline spin resonance between layers.
CRITERION #2: SUFFICIENT ORBITAL EXTENSION:
As was proposed above, the alignment of spin and field is not enough to effect a ferromagnetic resonance on its own. Since these are interactions between core electrons, their influence (and connections) need to be sufficiently enhanced and extended outward from the atomic cores in order to be felt by adjacent atoms in the crystal, and to facilitate ferromagnetic spin bonding.
In addition to cobalt’s 3 trigonal planar unpaired electrons, its 3rd shell also contains 5 di-electrons. (These are depicted by large black dots in the image below, and by the “5” in its center). Furthermore, 2 of the 4 di-electrons in the 2nd shell are aligned almost directly beneath two of the unpaired electrons of the 3rd shell. This should serve to constrict and extend the unpaired electron orbitals in a similar way to that proposed for iron. (While there may be less di-electron repulsion from the 2nd shell than in iron, there is more same-shell di-electron repulsion than in iron.)

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.Di-electron orbitals are larger than unpaired electron orbitals, and they have double the charge. They therefore repel unpaired electron orbitals more strongly. It has been proposed that this can not only constrict the unpaired electron orbitals but also cause them to become extended, radially outward, from the atomic core. In the case of cobalt, the unpaired electron orbitals are experiencing this form of constriction from 7 di-electrons — 5 from beside/around them in the same shell, and 2 from beneath them in the 2nd shell.
It is proposed that the combination of all this di-electron repulsion forces the influence of cobalt’s unpaired electron orbitals to be extended significantly further outward (just as a spinning cylinder, forced to become thinner, that responds by becoming longer). This extends the influence of these electrons’ spins and magnetic fields into the electron gas around the atomic cores, and it is suggested that this is what makes electron spin interactions between adjacent crystalline cobalt atoms possible. [Ref]
For more on the relative trend in ferromagnetic strength, see Magnetism & Magnetic Trends.
Curie Temperature & Structural Shift
Iron is by far the most strongly ferromagnetic of the three metals, yet cobalt has a higher Curie Temperature. This means that, even though iron is more strongly magnetic, cobalt (Co) can hang on to its magnetization to a much higher temperature. That must mean its ferromagnetic spin resonance is stronger than iron’s. How might we explain this?
According to the present proposed model, we might speculate that the reason for this is as follows. Cobalt contains three instances of ferromagnetic spin bonding (FSB) per atom, and the resonance is essentially two-dimensional, in the hexagonal crystal layers. Iron contains four instances of FSB per atom, and the resonance is three-dimensional and perfectly tetrahedral throughout the lattice. This causes iron to have a far more significant ferromagnetic spin resonance throughout its crystal, making it more strongly ferromagnetic than cobalt.
However, each of cobalt’s three instance of FSB involves 3 electrons holding each other in spin resonance. Each of iron’s four instances involve only 2-electron resonances. As such, cobalt’s spin bonding resonances are stronger and will therefore be able to withstand higher temperatures without thermalization disrupting them out of resonance. It is therefore proposed that this is what gives cobalt a higher Curie Temperature than iron, in spite of its weaker ferromagnetic strength.
THE STRUCTURAL TEMPERATURE SHIFT:
In terms of cobalt shifting from HCP to FCC when passing 450ºC, we might speculate that this is due to an additional (and weaker) spin resonance that can arise between layers.
As we mentioned above, HCP has columnar gaps that line up vertically (axially) through the crystal (as shown in figure 7 above). This could conceivably allow for the tri-electron spin-bonding resonances at three of the hexagonal corners to achieve an additional vertical (axial) element to their resonance. Since this resonance would occur between planes, across a larger crystal distance (than the tri-electron spin-bonding at the planar corners), it would be a weaker resonance. It might therefore only be able to attract the crystal into this structure at lower temperatures, when the disruption of thermalization is lower. In fact, the additional resonance may even be necessary in order to overcome greater di-electron repulsion, which may equally result from the presence of columnar inter-layer di-electron interactions.
We might further speculate that, when transitioning into FCC form, since the columnar gaps are no longer present, it is mainly the planar (equatorial trigonal) resonance that remains. The lack of columnar gaps should result in weaker (or shorter sets of) vertical electron interactions between layers, and this may therefore represent a state of lower di-electron repulsion. If so, it may cause the crystal to prefer this structure at higher temperatures in order to compensate for the instability due to thermalization.
PARAMAGNETIC 3d METALS: Scandium, Titanium, Vanadium, Chromium (also antiferromagnetic), Manganese
OTHER FERROMAGNETIC 3d METALS: Iron, Cobalt, Nickel
DIAMAGNETIC 3d METALS: Copper, Zinc
References:![]() See DeMystifySci Podcast: The Strange Behavior Of Humans And Magnets for a brief video discussion of this concept.
See DeMystifySci Podcast: The Strange Behavior Of Humans And Magnets for a brief video discussion of this concept.![]() A. Benn, J.G. Williamson, ‘pd-Hybridization And The Electron Geometry Of Fluorine, Neon And Iron’, Quicycle Journal (2024)
A. Benn, J.G. Williamson, ‘pd-Hybridization And The Electron Geometry Of Fluorine, Neon And Iron’, Quicycle Journal (2024)
J.G. Williamson, A. Benn, M. Rudolph, ‘Quantum Spin Coherence In 4 Derived 3-Spaces’, Quicycle Journal (2022)
RETURN to the Periodic Table

