Author: Arnie Benn
Protected: 60. Neodymium
Protected: 59. Praseodymium
Protected: 58. Cerium
Protected: 57. Lanthanum
56. Barium

RETURN to Periodic Table
Barium has an s-orbital di-electron in the 6th shell. It has the same electron configuration as strontium, but with five full shells within that have the identical configuration to xenon. Being one shell larger than strontium, barium has a slightly lower ionization energy and is therefore slightly more reactive and more soluble than calcium and strontium, allowing it to form the 2+ ion more readily.

 CLICK HERE to interact with this object
CLICK HERE to interact with this objectBarium’s outermost di-electron forms the 6th shell, finding these valence electrons further from (and more shielded from) the charge of the nucleus. We would therefore expect this di-electron to be less well-bound than the 5th or 4th shell di-electrons that we find in the outermost shells of strontium and calcium. The reason is that the 6th shell’s electron density fills a larger orbital volume. This should cause barium to be more paramagnetic — with a higher magnetic susceptibility (χm) value — than strontium or calcium above it on the periodic table. If electrons are further from the nucleus and less well bound, they will be more responsive to an external magnetic field. The other χm values on the periodic table reinforce this trend. But surprisingly, barium is less paramagnetic (with χm=+20) than both strontium (χm=+92) or calcium (χm=+40) above it.
This should either mean that the outer 6s2 di-electron is more well-bound than the di-electrons of the 5s2 and 4s2 orbitals, which seems unlikely, or that something else about the electron resonance of barium gives it added stability and coherence. This might arise from barium’s unique and highly symmetrical electron configuration, depicted below.
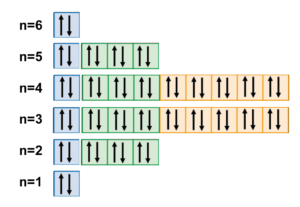
Interestingly, this symmetry seems to create a banded shell structure in the electron cloud. It has a virtual shell of highest electron density made up of the full 3rd and 4th shells, with decreasing electron density as we approach either the nucleus or the outer “boundary”.
An alternate view (shown below) of the orbitals shows an innermost 1st-shell sphere within a 2nd tetrahedral shell (dark blue) within a 3rd cubic shell (light blue) within a 4th cubic shell (brown) within a 5th tetrahedral shell (pink) within a 6s2 spherical di-electron (dark green).
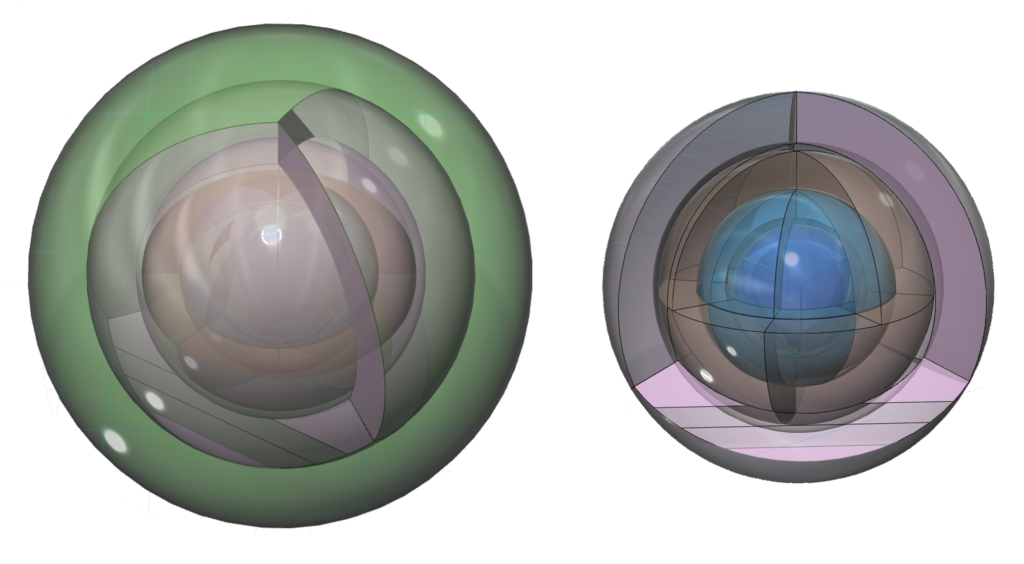
RETURN to the Periodic Table
OTHER GROUP II ELEMENTS: Beryllium, Magnesium, Calcium, Strontium, Barium
55. Cesium

RETURN to Periodic Table
Cesium has one electron in the 6th shell. It has the same electron configuration as rubidium, but with five full shells within that have the identical configuration to xenon. Being one shell larger than rubidium, cesium has a lower ionization energy and is therefore even more reactive than the metals above it when forming the 1+ ion. Pure cesium metal reacts explosively with water and readily self-ignites in air as it reacts with (and donates its valence electron to) oxygen.
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectAn alternate view (shown below) of the orbitals shows an innermost 1st-shell sphere within a 2nd tetrahedral shell (dark blue) within a 3rd cubic anti-prismatic shell (light blue) within an anti-aligned 4th cubic anti-prismatic shell (brown) within a 5th tetrahedral shell (pink) within an unpaired 6s1 electron (light green).

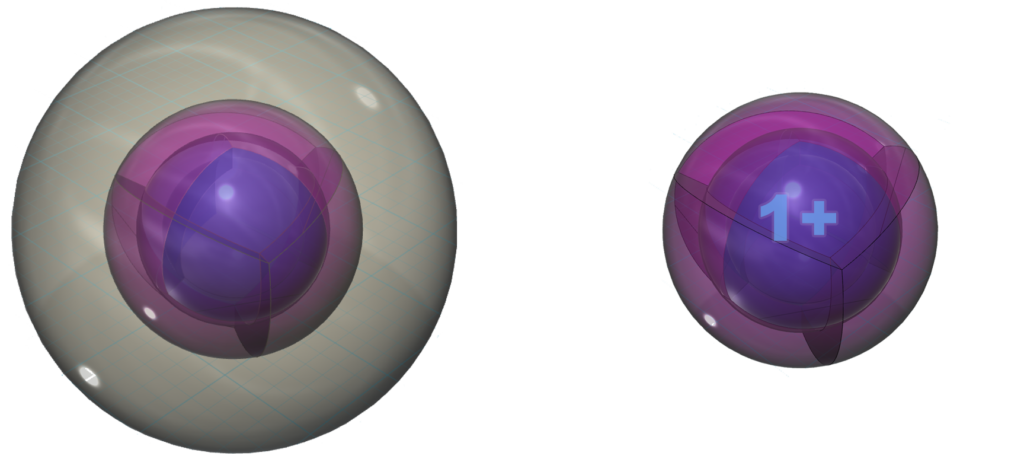
Ion formation
With a lower ionization energy than rubidium, cesium will give up its valence electron even more eagerly than rubidium in an ionic interaction, in order to reach the stability of the 5s25p6 noble gas configuration of xenon, which is a multi-di-electron state with five concentric full shells. That is why cesium forms a 1+ ionic state so violently.
Pure cesium metal is very soft, melting at around room temperature (~250C). It is so reactive that it is considered pyrophoric, which means that it ignites spontaneously in air, and even in very cold (-1160C) water, as it donates its valence electron to oxygen.
A fun video showing cesium metal and its reactivity can be found HERE.
Uses
Cesium has several industrial and manufacturing applications, but perhaps its most interesting one is its use as the element in atomic clocks that keeps time.
For an interesting video on how atomic clocks actually work, click HERE.
RETURN to the Periodic Table
OTHER GROUP I ELEMENTS: Lithium, Sodium, Potassium, Rubidium, Cesium
54. Xenon

RETURN to Periodic Table
Xenon is the sixth element with electrons in the 5p orbital. Xenon has five full shells. Like neon, argon, and krypton, it achieves symmetrical stability without the need to hybridize its 5s and 5p orbitals. It is not as unreactive as the other noble gases since its larger size means its electrons are further from and less well-bound to the nucleus. This lowers ionization energy to the point that it can bond with, for example, the electronegative oxygen and fluorine atoms. (The wireframe indicates the boundary of the n=5 shell.)
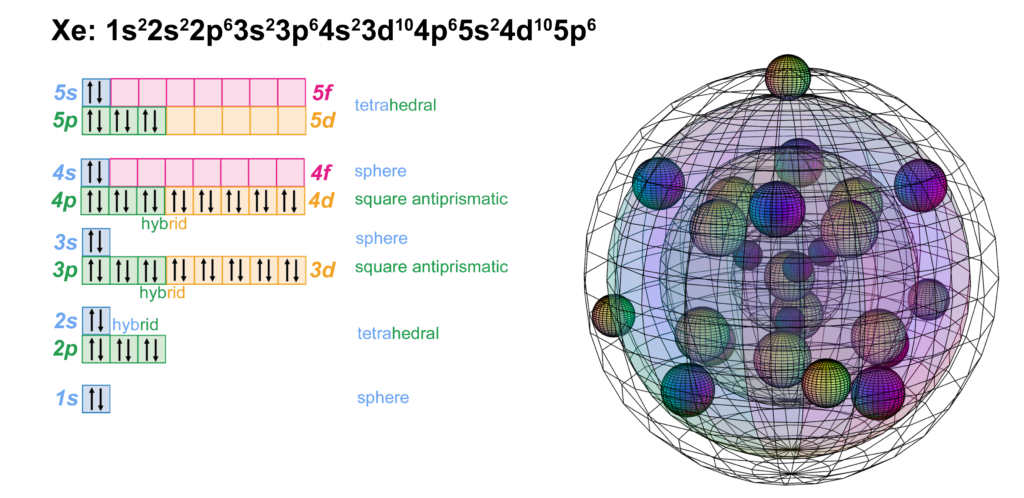
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectAn alternate view (shown below) of the orbitals of xenon shows an innermost 1st-shell sphere within a 2nd tetrahedral shell (dark blue) within a 3rd cubic anti-prismatic shell (light blue) within an anti-aligned 4th cubic anti-prismatic shell (brown) within a 5th tetrahedral shell (pink).
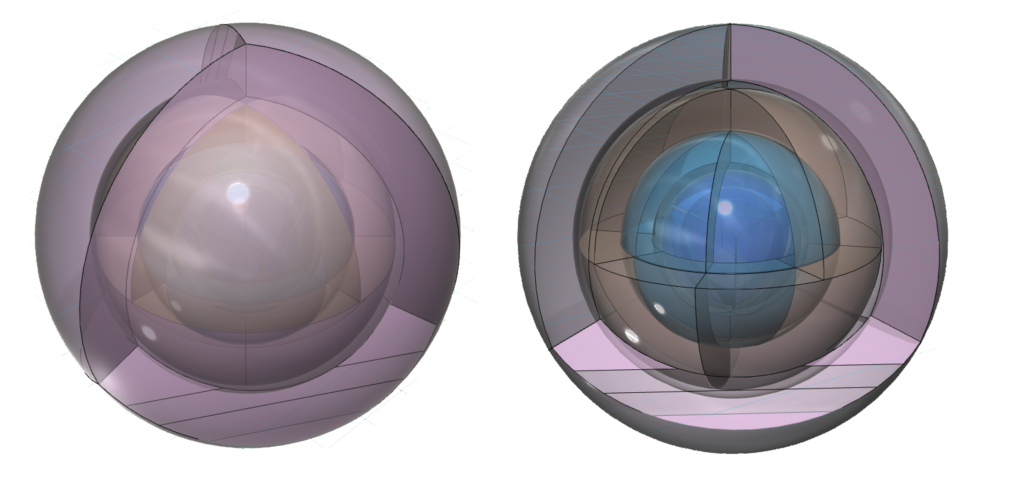
RETURN to the Periodic Table
SEE OTHER NOBLE GASES: Helium, Neon, Argon, Krypton, Xenon
53. Iodine

RETURN to Periodic Table
Iodine is the fifth element with electrons in the 5p orbital. It has the same electron configuration as bromine, just one shell larger. Like chlorine and bromine, iodine can support multiple possible molecular geometries, and can sustain multiply bonded atoms (with stronger electronegativity than iodine). (The wireframe indicates the boundary of the n=5 shell.)
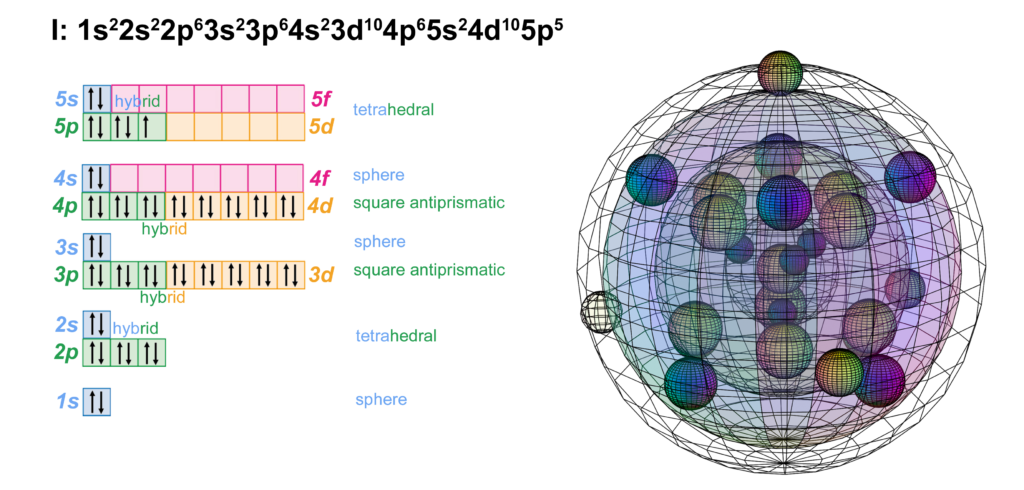
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectBonding & ion formation
Iodine is keen to obtain an extra electron to fill its fifth shell and it can bond with many atoms on the periodic table. Iodine can make one or more covalent bonds or gain an electron in ionic interaction in order to reach the stability of the 5s25p6 noble gas configuration of xenon, which is a multi-di-electron state with five concentric full shells. That is why iodine forms a 1– ionic state. The negative ion is larger than its neutral version because electrons now outnumber protons. This results in a lower effective nuclear charge — a lower average attraction by the nucleus on each electron.
RETURN to the Periodic Table
OTHER GROUP VII HALOGENS: Fluorine, Chlorine, Bromine, Iodine
