
RETURN to Periodic Table
Neon is the 10th element on the periodic table because it has 10 protons in its nucleus. The protons will attract 10 electrons to surround the nucleus in order to form a neutral atom. With 10 protons and 10 neutrons, most neon atoms have an atomic mass of 20 amu.
Electron Shell
Neon has two full electron shells — an inner core 1s shell, and a 2s shell containing a full p-orbital resonance. With 6 electrons in a 2p orbital, neon is believed to achieve stability with octahedral symmetry, and its 2s electrons can unhybridize and return to their preferred spherical di-electron state (see below). It is here proposed, however, that neon is more stable with a tetrahedral valence shell of 4 di-electrons (shown below. The large wireframe indicates the boundary of the n=2 shell).
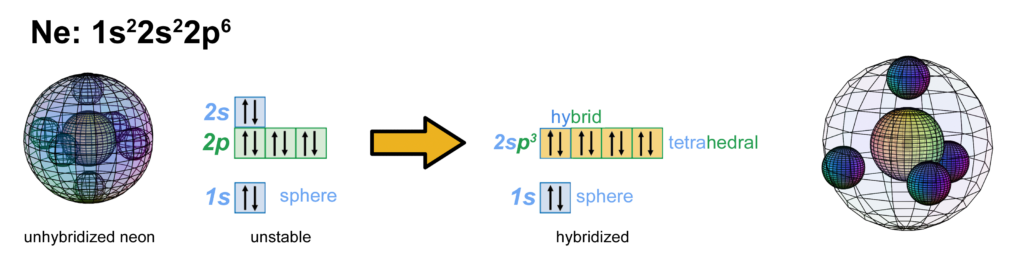
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: The small spheres in the image above simply indicate the directions of maximum electron density. The 2nd shell hybrid orbitals themselves are more like spherical tetrahedra that fill the volume within their shell with electron density. It will be highest at the center of the face of each orbital (as in the traditional sp3 lobe shapes) and will decrease toward the nodal regions between orbitals — as wave structures usually do — where electron density will be lowest (though not zero).
NOTE ALSO: Even though it is often useful to talk about these orbitals as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in this harmonic wave structure.
The diagram below shows only neon’s four 3rd shell sp3-hybrid orbitals. It is proposed that each of these hybrid orbitals contains two electrons in the (bosonic) di-electron state.
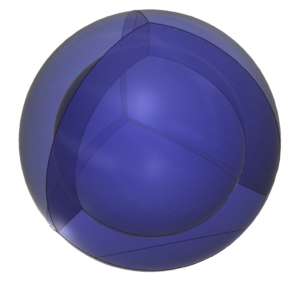
Intuitively, it seems more stable to consider the electron structure of neon (and of the full n=2 shell) as a tetrahedral arrangement of four di-electrons since it involves a greater degree of field cancellation than a full p-orbital with a single electron occupying each lobe. [Ref]
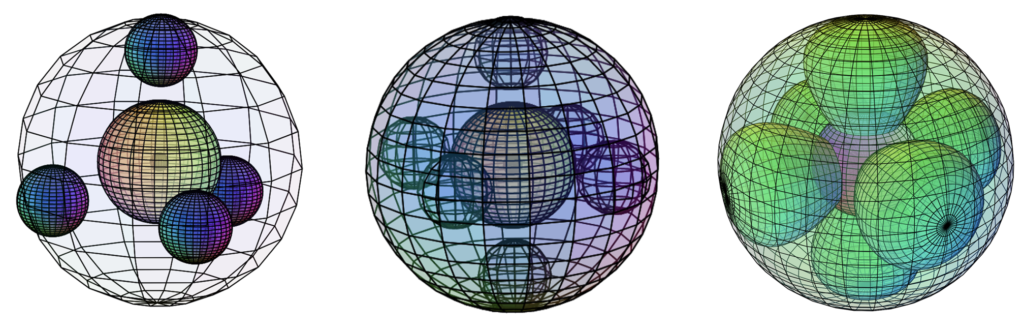
In either case, its high degree of symmetry, with all electrons paired, renders neon, like helium, unavailable to bond, and therefore chemically unreactive. It is a gas under normal conditions, and it is called a ‘noble’ gas because of its non-interaction.

Neon has a smaller atomic radius than fluorine. (This square represents the size of the largest atom).
Its small size results from its high effective nuclear charge. It has no electronegativity, given that it has a full electron shell, and it will therefore neither gain nor lose electrons.
Unlike the other elements of the second row, neon’s non-bonding (non-reactivity) makes it ‘completely’ non-metallic.
In the case of argon (Ar), since there are two concentric tetrahedral shells, they will align to form an antiparallel nested tetrahedral geometry because this minimizes repulsion between layers. The place of lowest electron density — where the nodal vertices intersect — on one shell is set against the highest electron density at the center of a face on the adjacent concentric shell. This is therefore the lowest energy state that nested tetrahedral shells can achieve.
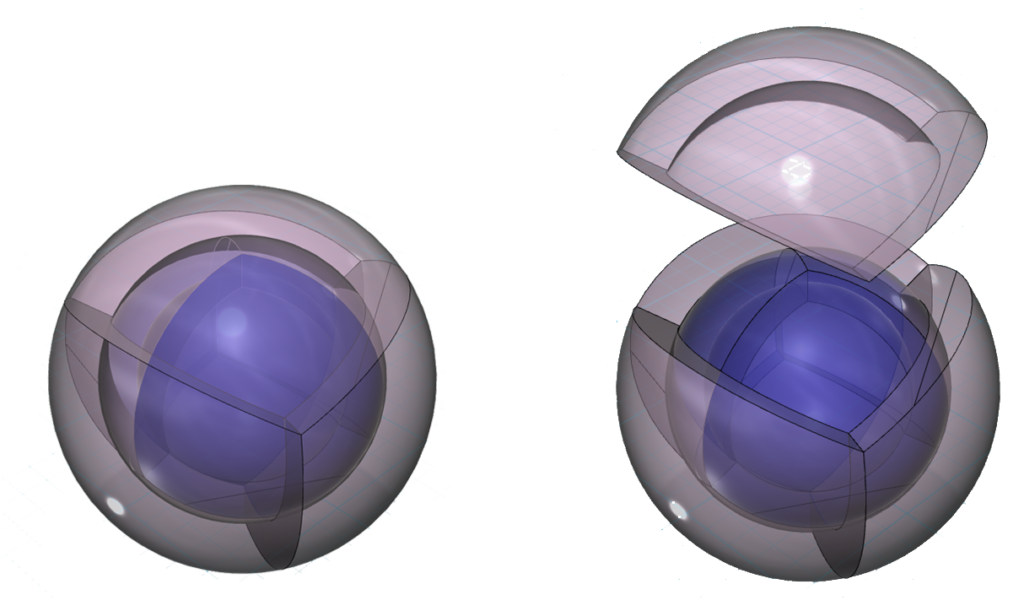
 CLICK HERE to interact with this object
CLICK HERE to interact with this objectUses
One of its primary uses (along with other noble gases) is as a gas in lighting (glow discharge) tubes, and it gives neon signs their characteristic bright red color.
RETURN to the Periodic Table
SEE OTHER NOBLE GASES: Helium, Neon, Argon, Krypton, Xenon
