RETURN to Periodic Table
Below: The 1st Element, Electrostatic Attraction, Electron Shell, Ion Formation, Dihydrogen, Importance, Isotopes, Delving Deeper, Electroproton
The 1st Element
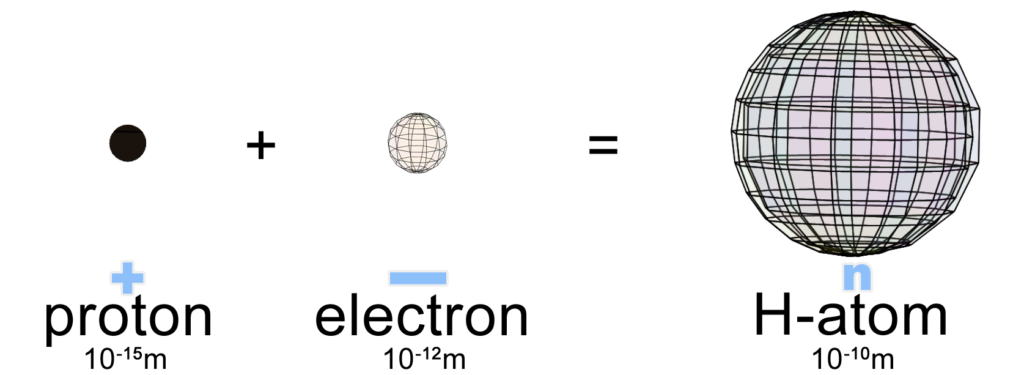
Hydrogen is the 1st and simplest element on the periodic table because it has only 1 proton at its center (in its nucleus). Since the proton carries a positive charge, it will attract 1 electron to it in order to balance its charge because electrons have a negative charge. Hydrogen therefore forms a neutral atom when a single electron surrounds and envelops a single proton with a sphere-shaped cloud of charge density. Within the boundary of this cloud, the charges balance perfectly.
Electrons are ignored when considering the mass of the atom because they are over 1,800 times lighter than protons and neutrons. With only 1 proton and no neutrons, hydrogen has an atomic mass of 1 ‘atomic mass unit’ (amu).

NOTE: An electron does not have a fixed size! It will occupy much less volume when it is isolated than when it is surrounding a nucleus, and far more volume when in the solid state. (See Understanding Electrons for more detail.) Note also that the sizes depicted in the image above are not to the scale reflected in the numbers. An isolated proton is three orders of magnitude (103 times) smaller than an isolated electron. (Higher energy means smaller size in quantum mechanics. See De Broglie wavelength.) Furthermore, in a hydrogen atom, the electron occupies a volume orders of magnitude greater than when it is isolated.
Electrostatic Attraction 
The reason that opposite charges attract each other is that, between them, their electric fields are aligned in precisely opposite directions. This causes their fields to cancel through destructive interference, which lowers the energy between them. Since lower energy is desirable, they are attracted into the direction of this state. (ref) That is why the hydrogen atom is so much larger than a proton plus an electron. Since energy has been drained from the system by their mutual charge neutralization, the system increases in size. (Lower energy means larger size in quantum mechanics.)
Like charges repel because their fields are aligned in the same direction. This increases field energy between them through constructive interference, and they are forced away from this undesirable, higher-energy state.
Electron Shell 
Hydrogen only has 1 electron shell. Its single electron forms a spherical orbital called an s-orbital. We can therefore describe hydrogen as having an electron configuration of 1s1. The “1–s–1” means: shell number 1, the s-orbital, which contains 1 electron. (see image below)

 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.NOTE: Even though it is often useful to talk about electron orbitals (and even subatomic particles for that matter) as separate, they are all — the entire atom is — part of a single, coherent, harmonic, resonant, phase-locked, spherically-symmetrical quantum wave state, and it is all electromagnetic at the root-energy level. Orbitals and their ‘boundaries’ can be seen as nothing more than nodes and antinodes in a single harmonic wave structure.
A particle is a perfectly-struck electromagnetic ‘note’; an atom is a perfectly-struck electromagnetic harmony between these electromagnetic ‘knots’.
The image below provides a different view of the spherical hydrogen atom. The size of the nucleus in the center is greatly exaggerated. If the electron cloud were the size of a large football stadium, the nucleus would be the size of a penny at the center of the field — barely visible.
As we saw above, an isolated proton is 100,000 times smaller than a hydrogen atom’s electron cloud. (As we will see below, though, a quantum view might paint a slightly different picture of the sizes of the proton and electron when in a mixed, atomic state.)

Hydrogen has the second smallest atomic radius on the periodic table. (This square represents the size of the largest atom, Francium, Fr).
Hydrogen’s electronegativity value makes it a non-metal, though with some slight metallic character, given that it can give an electron away in certain situations. (For example, it has the same electronegativity value as palladium, Pd.)
The fact that hydrogen has only one electron cancelling charge and field with the nuclear proton makes hydrogen particularly keen to keep that electron. This gives hydrogen a high ionization energy (1,312 kJ/mol = 13.6 eV), meaning that it is difficult for other atoms to steal its electron. Though it does occur, for example, when the hydrogen ion (H+) forms, which makes acid. (See below)
Hydrogen is also quite reactive in search of another electron to pair with its single electron. When two electrons pair, they form a di-electron state, which is a much more stable and desired state than an unpaired electron. (See Understanding Electrons) This occurs, for example, when the hydride ion (H–) forms, which makes base (also known as alkali). (See below)
Ion Formation 
An ion is an atom that either has more or fewer electrons (–) than protons (+). If they are not present in equal amounts, the charges are not in balance, and the atom carries an overall charge.
Electron pairing and electron symmetry are such favorable energy states that they will happen even at the expense of the atom having a neutral charge. Some atoms easily form ions by gaining or losing electrons in order to achieve full, symmetrical electron shells. This stabilizes them more than having the same numbers of protons and electrons.
In the presence of more electronegative non-metal atoms (like chlorine), hydrogen can lose its electron and the (acidic) hydrogen ion (H+) can form (below, left). The stronger an acid, the more H+ ions it produces in solution. An H+ ion is an exposed proton, which is why strong acids can be so corrosive. Each H+ ion is desperately seeking an electron in which to clothe its bare proton nucleus. It does so by taking an electron from another substance around it, which results in a (sometimes violent) chemical reaction.
In the presence of metal atoms (like sodium), hydrogen can attract an extra electron and the (alkaline) hydride (H–) ion can form (below, right). This occurs because metal atoms have low ionization energies, meaning that their outer electrons can become delocalized or easily removed by electronegative non-metal atoms (like hydrogen, oxygen, or chlorine).

The acidic hydrogen (H+) ion (left), neutral hydrogen (H) atom (center), and alkaline/basic hydride (H–) ion (right). The wireframe with light coloring represents a single electron; the full-color wireframe represents a di-electron.
Dihydrogen Molecule (H2) 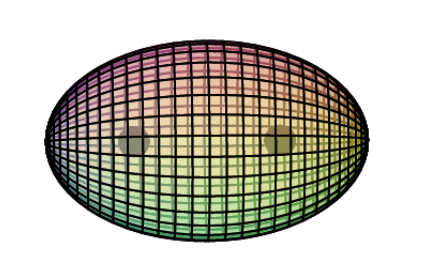
When two hydrogen atoms bond to form an H2 molecule (shown below), the two bonding electrons (with opposite spins) form a single di-electron state. This is a different state than two single electrons. It is a boson state where the two electron wave functions are completely superimposed upon one another for maximum magnetic field cancellation. This binds the two nuclei together in a covalent bond.
The electron density will not be evenly distributed. It will be more dense between the nuclei because that is where the most positive charge requires neutralizing. It will be less dense on the outsides, but never zero because nuclear charge must be cancelled symmetrically in all directions around a nucleus. Overall, the electron density keeps the protons suspended within it, although they remain a distance apart because protons repel each other.
Conceptually, at the atomic level, a molecule is therefore very much like a discrete metallic crystal — positive cores suspended within a 3D ‘electron gas’ — though, in this case, a ‘crystal’ with a limited, fixed extent.
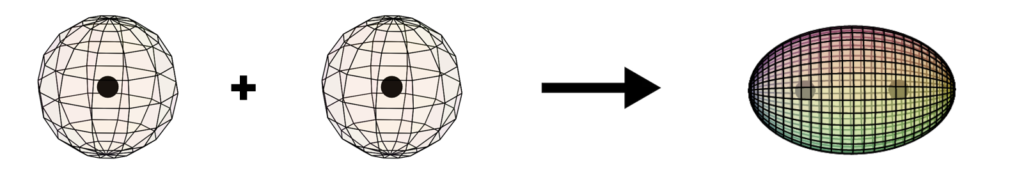 Formation of the dihydrogen (H2) molecule
Formation of the dihydrogen (H2) molecule
 CLICK HERE to interact with this object.
CLICK HERE to interact with this object.We also see di-electron formation in an individual helium (He) atom, although it is a spherical di-electron (making up its 1st shell), and it contains one nucleus with two protons rather than two nuclei with one each.
Hydrogen’s Importance
Hydrogen is important for many reasons. Some of these include:
It is the most abundant element in the universe because the hydrogen atom is the simplest atom — 1 proton + 1 electron.
Hydrogen makes up stars, which fuse the hydrogen atoms together to form helium atoms, resulting in a large release of heat energy — fusion energy. As stars go through their stellar life cycles, they forge the heavier elements from the smaller elements. Stars are literally the matter-making machines of the universe, and hydrogen is the raw material.

Hydrogen (H2) combines with oxygen (O2) to form water (H2O), the most essential compound for sustaining life on Earth. When hydrogen and oxygen combine, a large amount of energy can be released as heat because the product, H2O, is a much more stable and favored arrangement of atoms than the elemental gases individually. This oxygen-combining reaction is called a combustion reaction because it often involves enough heat to cause burning.

Burning is the chemical process that occurs when a substance combines with oxygen, releasing a lot of heat. It is the heat from this combustion reaction that is used to power the rocket engines of the space shuttle, for example, when liquid oxygen and liquid hydrogen are used as the fuel. This is a very clean-burning fuel since water vapor is the “waste” product.
2H2 + O2 —> 2H2O + explosive heat
The formation of water from hydrogen and oxygen is a reaction that can also be reversed. Hydrogen and oxygen can be obtained by splitting water molecules apart. The most common way to do this is by electrolysis — using electricity to split the water. (The energy input is required because the atoms are being rearranged into a less favored arrangement.)
Electricity + 2H2O —> 2H2 + O2
The reverse is also true. Electricity can be obtained by allowing hydrogen and oxygen to react in a non-explosive way. This is what occurs in a hydrogen-oxygen battery, known as a hydrogen fuel cell.
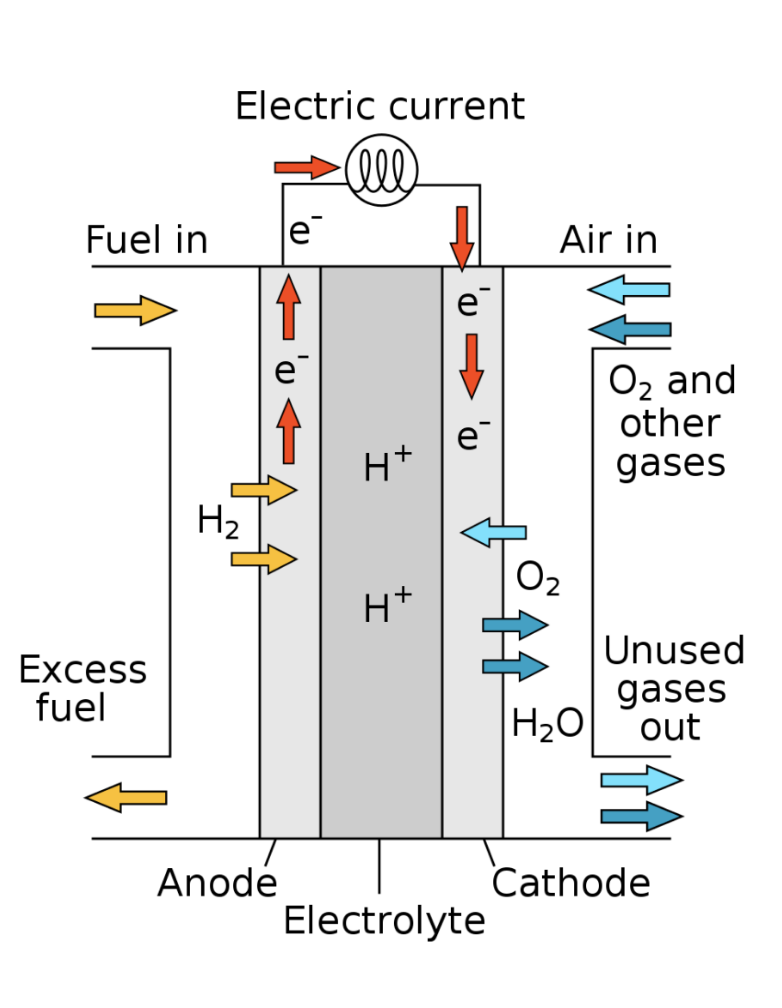
2H2 + O2 —> Electricity + 2H2O
On space vehicles and space stations, these two processes are both used, and one can be used to supplement the other. Water can be turned into oxygen for breathing if needed, but it also consumes electricity. Oxygen can be turned into water if needed, which also produces electricity. Or oxygen can be turned into electricity if needed, which also produces water. And hydrogen is an essential part of these processes.
(Images: Wikimedia commons)
Hydrogen Isotopes
While most hydrogen atoms contain 1 proton and 1 electron, there are some that also contain 1 or 2 neutrons. Atoms that have the same number of protons are the same element, but if they have different numbers of neutrons, they will have different atomic masses. These are called isotopes. There are three isotopes of hydrogen.
Protium ![]() (1H): 99.98% of all hydrogen atoms contain 1 proton (and 1 electron), and have an atomic mass of 1. (Electrons do not account for a meaningful amount of the atom’s mass.)
(1H): 99.98% of all hydrogen atoms contain 1 proton (and 1 electron), and have an atomic mass of 1. (Electrons do not account for a meaningful amount of the atom’s mass.)
Deuterium ![]() (2H): A small percentage of hydrogen atoms contain 1 proton and 1 neutron in the nucleus, and have an atomic mass of 2.
(2H): A small percentage of hydrogen atoms contain 1 proton and 1 neutron in the nucleus, and have an atomic mass of 2.
Tritium ![]() (3H): A small percentage of hydrogen atoms contain 1 proton and 2 neutrons in the nucleus, and have an atomic mass of 3.
(3H): A small percentage of hydrogen atoms contain 1 proton and 2 neutrons in the nucleus, and have an atomic mass of 3.
Water made with deuterium — called “heavy water” (D2O or 2H2O) — has a density about 11% higher than regular water (H2O). It has various properties that differ slightly from regular water, and it has typically been used in nuclear reactors.
![]() DELVING DEEPER:
DELVING DEEPER:
We normally represent atoms as having a very small nucleus and a much larger electron shell. This is not inaccurate as a free proton has a DeBroglie wavelength that is 3 orders of magnitude smaller than the electron, though its mass-energy is 3 orders of magnitude larger. (This is because size and energy are inversely proportional.) It is interesting to note that a “quantum” representation of the hydrogen atom, as shown below, might show the proton and electron as superimposed and the same size. Not only do the electron and proton in the hydrogen atom assume the same 1-Angstrom DeBroglie wavelength (indicated below, which alludes to their size), both of their charge fields fill the entire sphere of the atom, even though both increase in density towards the center (as is manifest in scattering experiments). This underscores that the hydrogen atom is not only a different type of quantum state than a proton and an electron, it is a single harmonic quantum state of two particles in a spherically symmetrical, resonant embrace.
 A quantum-state view of the hydrogen atom’s formation
A quantum-state view of the hydrogen atom’s formation
Another way to understand the larger size of the atomic resonance is that energy has been lowered in the system by virtue of the total electric field (charge) cancellation between the electron and proton. Lower energy equates to larger size, just as smaller size attests to larger energy. The single atomic electron’s charge cloud is now 100 times the size of the isolated electron.
Outside the boundary of the hydrogen atom, its charge tends to zero, though its electron and proton spins remain detectable and can interact.
Hydrogen vs Neutron
The hydrogen atom contains 1 proton and 1 electron. Since their energies, charges, and fields are completely inter-acting, we might call the hydrogen atom an electro-proton.
Like the hydrogen atom, a neutron is neutral because it contains the charge of the proton plus the charge of the electron. The mass of the neutron (939.6 MeV) is very similar to (though slightly larger than) the combined masses of the proton (938.3 MeV) and electron (0.511 MeV). The difference in their masses represents the difference in energy between the neutron state and the electro-proton state. This energy is given off when a neutron decays.
While a neutron is stable when in harmony with protons (and neutrons) in a nucleus, an isolated neutron is not a stable particle. It usually decays in less than 15 minutes, splitting into a proton, an electron, and emitting the additional energy (in the form of an antineutrino).
In the electro-proton, two particles are in resonance with one another, engaged in a harmonic dance that exists in a perfect balance between charges. In the neutron, the proton and electron structures have merged into one new resonance topology — a single new coherent energy flow.
OTHER GROUP I ELEMENTS: Hydrogen, Lithium, Sodium, Potassium, Rubidium, Cesium
RETURN to Periodic Table