
RETURN to Periodic Table
The elements of Group VII are called the Halogens. When paired with hydrogen, the halogens become acids, like hydrochloric acid (HCl). This is because, when they react with water, they release the hydrogen as an H+ ion, forming an acidic solution.
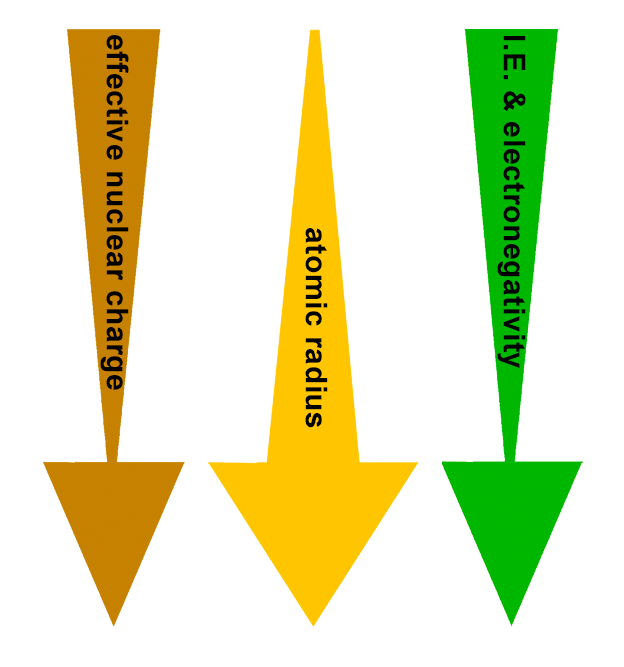
PERIODIC TRENDS:
Effective nuclear charge (Zeff) decreases down the group.
Therefore:
Atomic radius increases down the group.
Ionization energy decreases down the group.
Electronegativity decreases down the group.
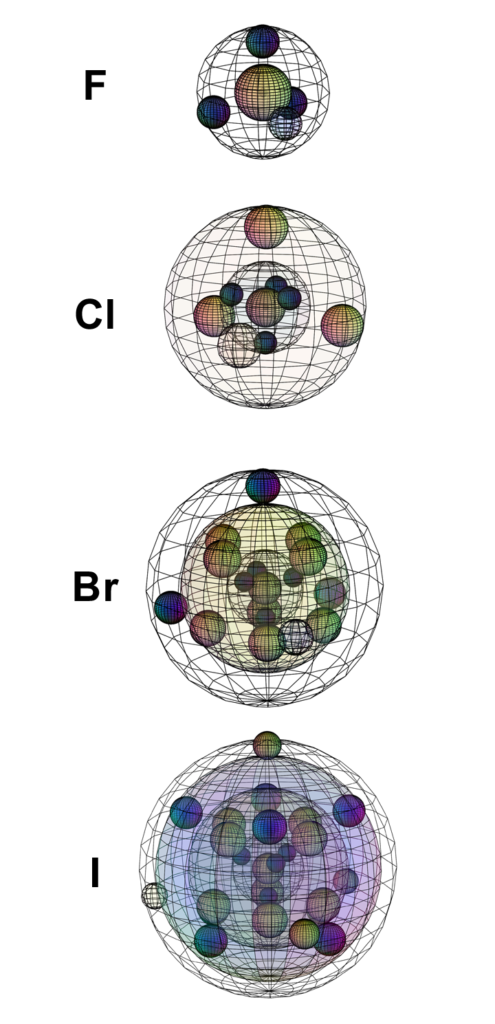
The elements in Group VII are 1 electron away from a full valence shell, and they can therefore be quite reactive in seeking to gain an electron to form the 1– ion. As we move down the seventh group of the periodic table, atomic radius increases as new shells are added. Valence electrons are therefore further from the attraction of the nucleus. The larger the atom, the smaller becomes its electronegativity and ionization energy, and the less reactive it is.
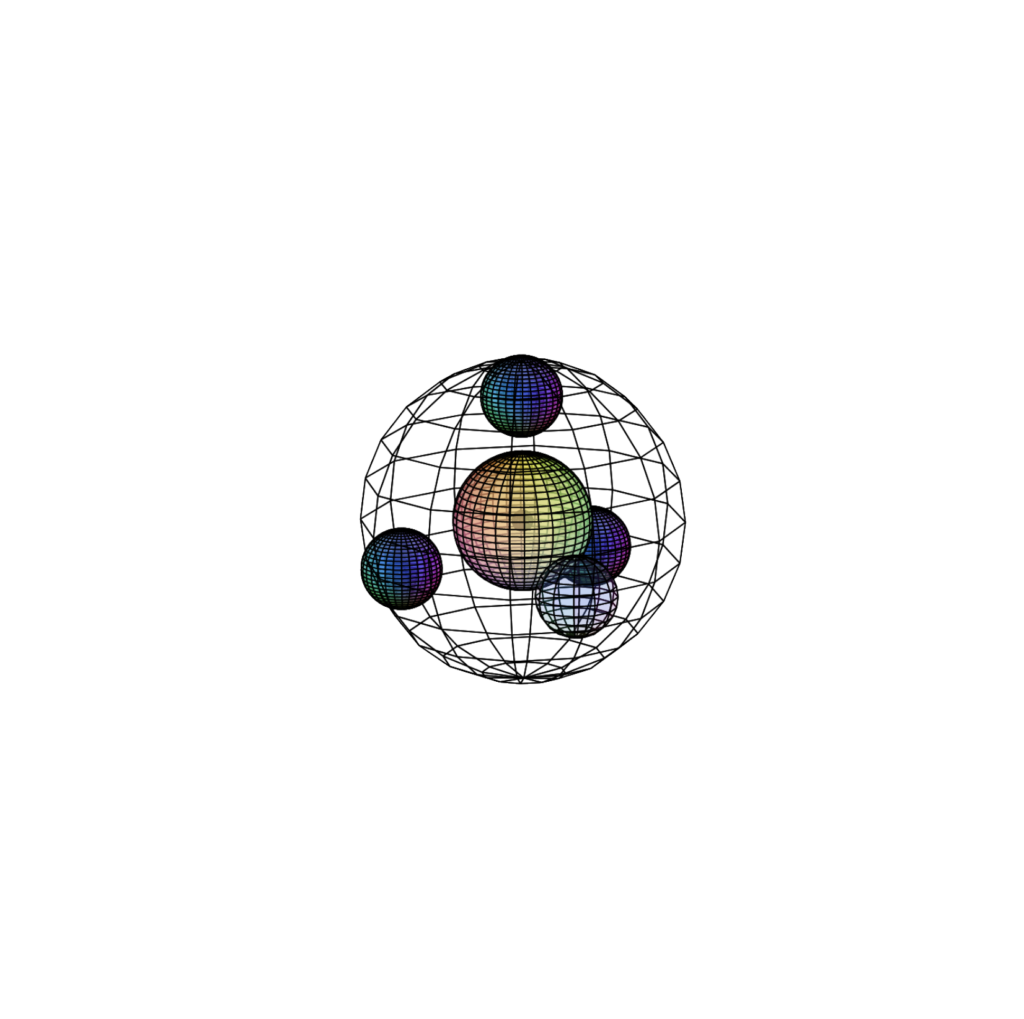
FLUORINE (9F)
Fluorine’s bonding atomic radius is only 57 pm, almost twice as small as chlorine. As such, its high effective nuclear charge is far more influential. In addition, fluorine is 1 electron short of having a full 2nd shell. This gives it a high ionization energy and makes it extremely (and often dangerously) reactive in seeking another electron. Since it is also the smallest atom to have an almost filled p-orbital, fluorine has the highest electronegativity (χe=3.98) on the periodic table. It can only make one covalent bond, but it pulls electrons so strongly that it can make it with just about every other atom on the periodic table. Elemental fluorine (F2) is a gas at room temperature because the molecule is non-polar.
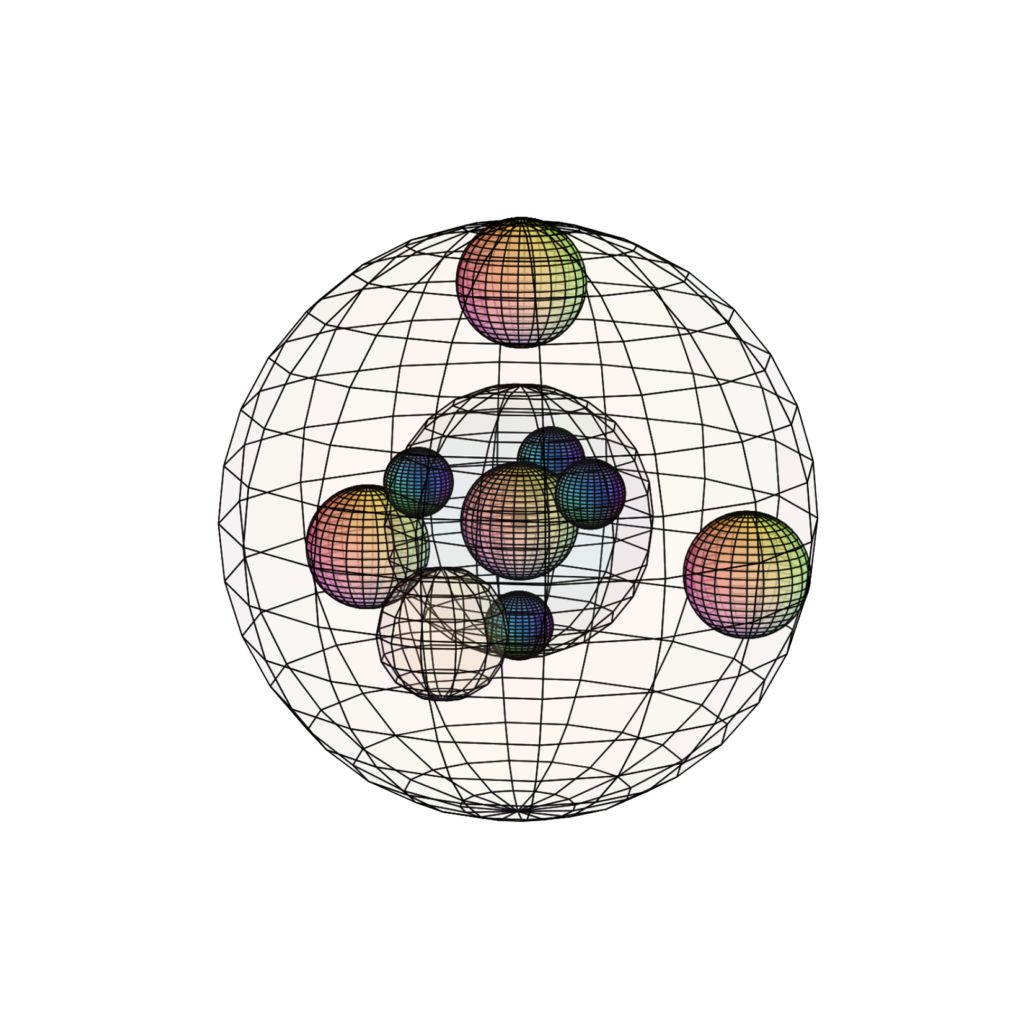
CHLORINE (17Cl)
Chlorine is 1 electron short of having a full 3rd shell. This gives it a high electronegativity (χe=3.16) and ionization energy, though not as high as fluorine. Chlorine’s bonding atomic radius is 102 pm, almost double the size of fluorine, and hence its lower electronegativity. Chlorine is very reactive in seeking another electron, and while it usually makes 1 covalent bond, it can make up to 7 bonds when bonding with more electronegative atoms like oxygen or fluorine. This is because the larger 3rd shell can accommodate more electron density than the 2nd shell, and can therefore sustain more than 4 electron domain (bonding) directions. Elemental chlorine (Cl2) is a gas at room temperature because the molecule is non-polar.

BROMINE (35Br)
Bromine is 1 electron short of having a full 4th shell. This gives it a high electronegativity (χe=2.96) and ionization energy, though not as high as chlorine. Bromine is reactive in seeking another electron, and while it usually makes 1 covalent bond, it can make up to 7 bonds when bonding with more electronegative atoms like oxygen or fluorine. Elemental bromine (Br2) is a liquid at room temperature. Although the molecule is non-polar, it has enough electrons that London dispersion forces become strong enough to pull the molecules close enough together to form the liquid state. Bromine’s bonding atomic radius is 120 pm, only about 20% larger than chlorine.
IODINE (53I)
Iodine is 1 electron short of having a full 5th shell. This gives it a high electronegativity (χe=2.66) and ionization energy, though not as high as Bromine. Iodine is somewhat reactive in seeking another electron, and while it usually makes 1 covalent bond, it can make up to 7 bonds when bonding with more electronegative atoms like oxygen or fluorine. Elemental iodine (I2) is a solid at room temperature. Although the molecule is non-polar, it has enough electrons that London dispersion forces become strong enough to pull the molecules close enough and hold them in place to form the solid crystalline state. Since these are relatively weak forces, solid iodine evaporates easily. Iodine’s bonding atomic radius is 139 pm, less than 20% larger than bromine.
ASTATINE (85At) is not shown here.
PERIODIC TRENDS: PERIOD II, PERIOD III, GROUP I, GROUP II, GROUP VII, GROUP VIII
RETURN to the Periodic Table
