
RETURN to Periodic Table
Each element in the 3rd period (or row) of the periodic table is larger than the element above it (in the 2nd period) since the 3rd period elements have an additional electron shell.

PERIODIC TRENDS:
Effective nuclear charge (Zeff) increases across the period.
Therefore:
Atomic radius decreases across the period.
Ionization energy increases across the period.
Electronegativity increases across the period
As we move across the 3rd period, elements become smaller. This occurs because the next element in the row, while in the same shell, has one more proton and one more electron than the element to its left. This means there is a greater inward attraction between the more positive nucleus and the more negative electron cloud, which is called effective nuclear charge (Zeff), and this shrinks the size of the atom somewhat.
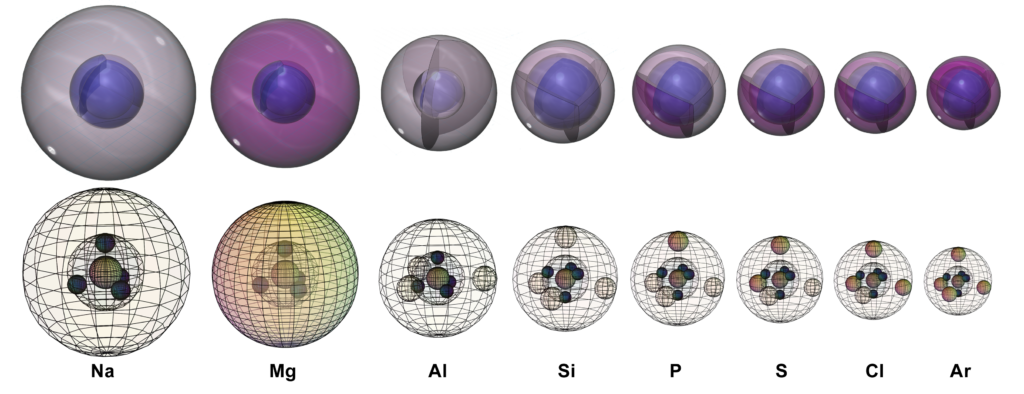
As a result of the increasing Zeff, each element in the period has a higher electronegativity than the element before it. The ionization energy also generally increases across the period, although there are exceptions. As we see in the graph below, which highlights the elements of this 3rd period, magnesium and phosphorus (in blue) have values that are larger than their neighbors. This is due to the added stability of their electron structures, as we saw with beryllium (Be) and nitrogen (N) in the 2nd period. We also see that ionization energies continue to decrease as we proceed from one period to the next.
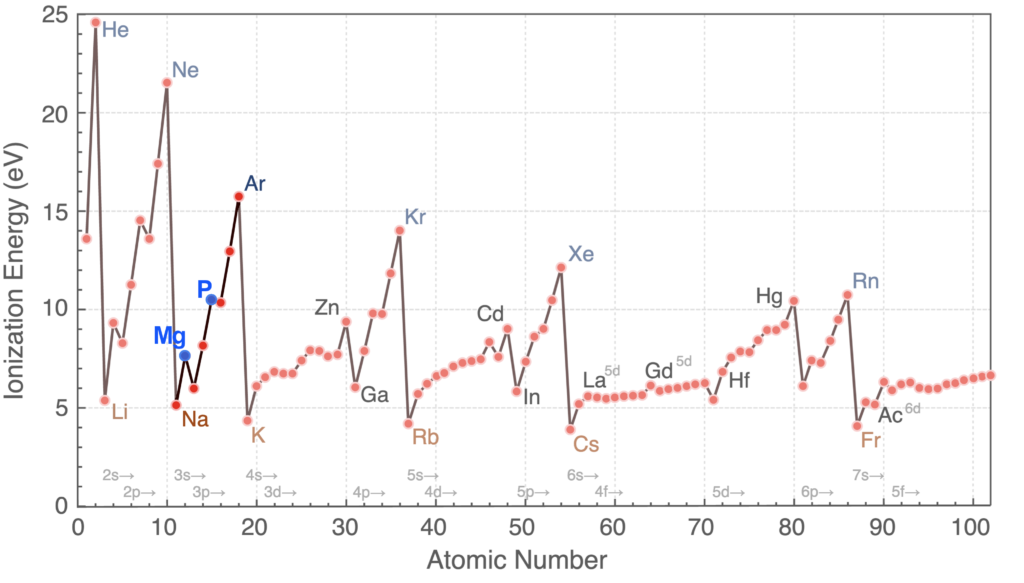
The first elements in the row are most willing to lose their electrons to achieve a full 2nd electron shell, but as we proceed further along and the atoms gain electrons in the 3rd shell, they increasingly want to gain electrons to fill the 3rd shell.
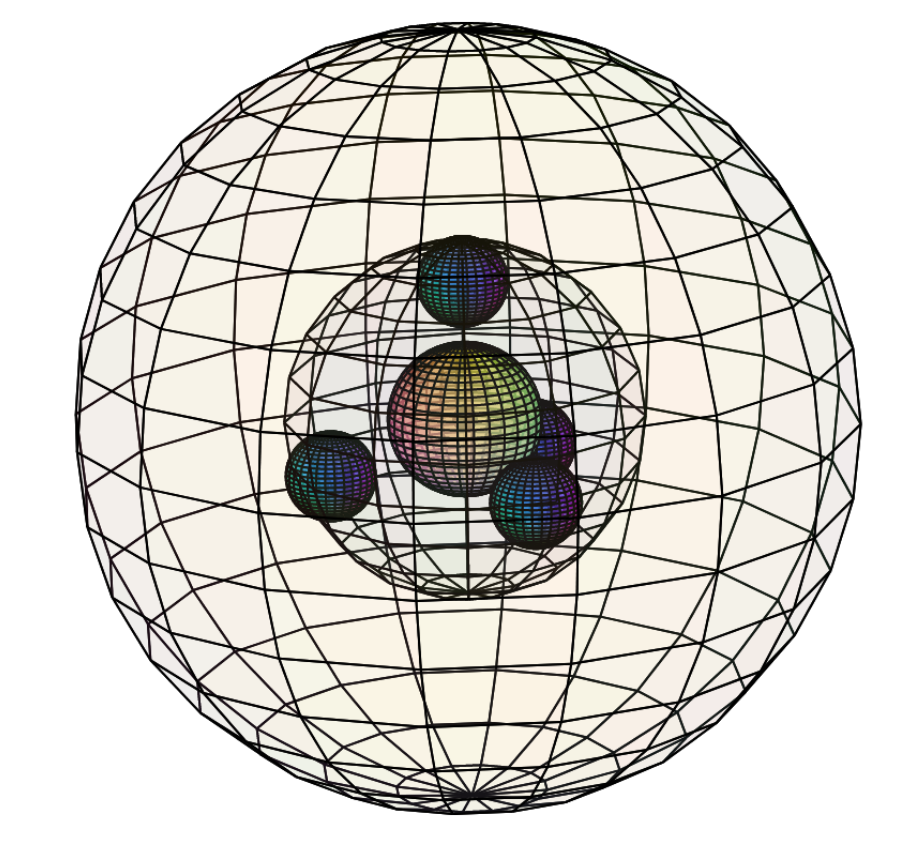
SODIUM (11Na)
Sodium is an alkali metal, and is the largest atom in the 3rd period with a bonding atomic radius of 180 pm. Its 3s1 electron occupies more space than a 2s2 di-electron and is further away and more shielded from the attraction of the nuclear charge. This makes sodium much more willing to lose its valence electron in search of a full shell configuration. This results in sodium having a lower electronegativity (χe=0.93), a lower ionization energy (496 kJ/mol), and a much higher reactivity than lithium above it in the 2nd period. When placed in water, sodium react so readily and releases so much heat energy in the process that it spontaneously ignites the hydrogen gas that is also released in the reaction. Given the similarity of their single valence electron configurations, sodium is only slightly more paramagnetic (χm=+16) than lithium, which means that it is attracted to a magnetic field because its unpaired outer electron can align with an external magnetic field.
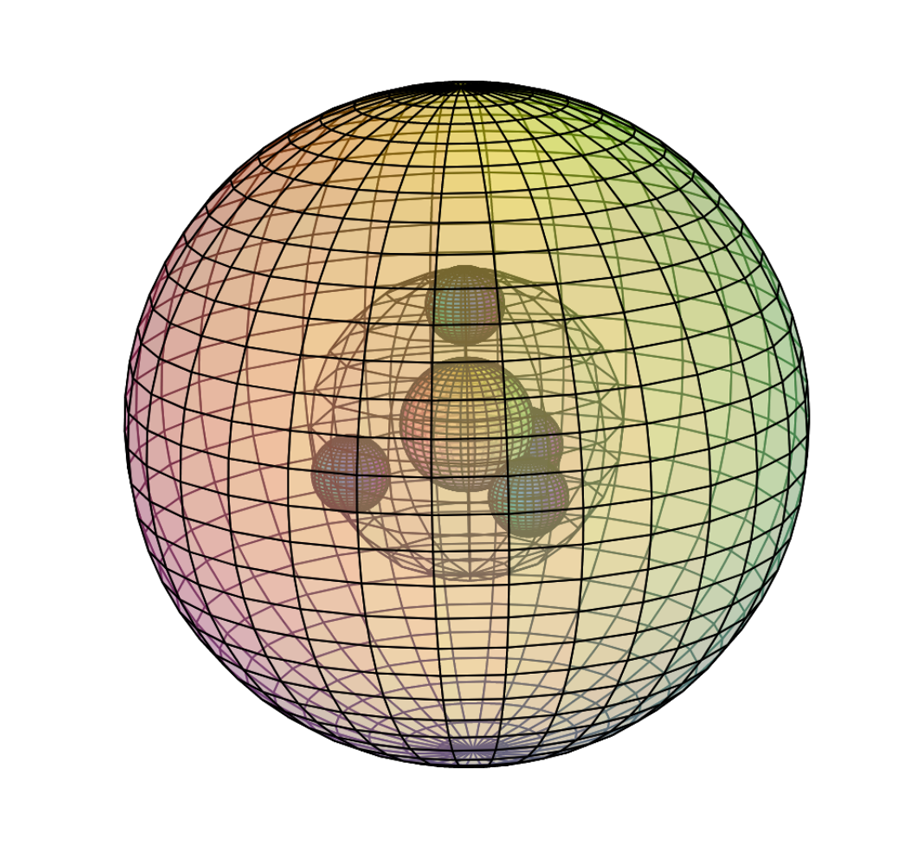
MAGNESIUM (12Mg)
Being in Group II, magnesium is an alkali earth metal. It has a stable 3s2 di-electron, and is therefore less reactive than sodium to its left. Magnesium is significantly larger than beryllium and its 3s2 di-electron therefore occupies more space than a 2s2 di-electron. Since magnesium has a higher effective nuclear charge (Zeff) than sodium, it is smaller, with a bonding atomic radius of 145 pm. Since the outer electrons are closer to the attraction of the nucleus, magnesium has a higher electronegativity (χe=1.31) and a higher ionization energy (738 kJ/mol), making it slightly less reactive than sodium. Magnesium is also paramagnetic (χm=+13.1), which means that it is attracted to a magnetic field because its outer electrons can align individually with an external magnetic field.

ALUMINIUM (13Al)
Aluminium experiences a similar orbital hybridization to boron, resulting in a triangular (trigonal planar) geometry of unpaired electrons. As a result, its ionization energy (578 kJ/mol) is not quite as high as that of magnesium. Aluminium has a higher effective nuclear charge than magnesium, and the stronger inward attraction makes it a smaller atom than magnesium, with a bonding atomic radius of 125 pm. This gives it a higher electronegativity (χe=1.61), less reactivity, and a more metallic nature than magnesium. It is paramagnetic (χm=+16.5), which means that it is attracted to a magnetic field because its unpaired outer electron can align with an external magnetic field.

SILICON (14Si)
Silicon experiences a similar orbital hybridization to carbon, resulting in a tetrahedral (4-directional) geometry of unpaired electrons. As a result, its ionization energy (786 kJ/mol) is higher than that of aluminium (and even than magnesium) since it has 4 electrons in a stable and spherically-symmetrical resonance structure. Silicon has a higher effective nuclear charge and a stronger inward attraction, with a bonding atomic radius of 110 pm. Since it has a higher electronegativity (χe=1.90), silicon becomes more powerful at attracting electrons than aluminium, though not as powerful as the smaller carbon atom in the 2nd period above it. This makes silicon the first semi-metal atom in the 3rd period, giving it unique (semi-conducting) properties as a result. Silicon is diamagnetic (χm=–3.12), which means that it is repelled from a magnetic field because its outer electrons are all paired (in covalent bonds), and they can therefore not align with an external magnetic field.
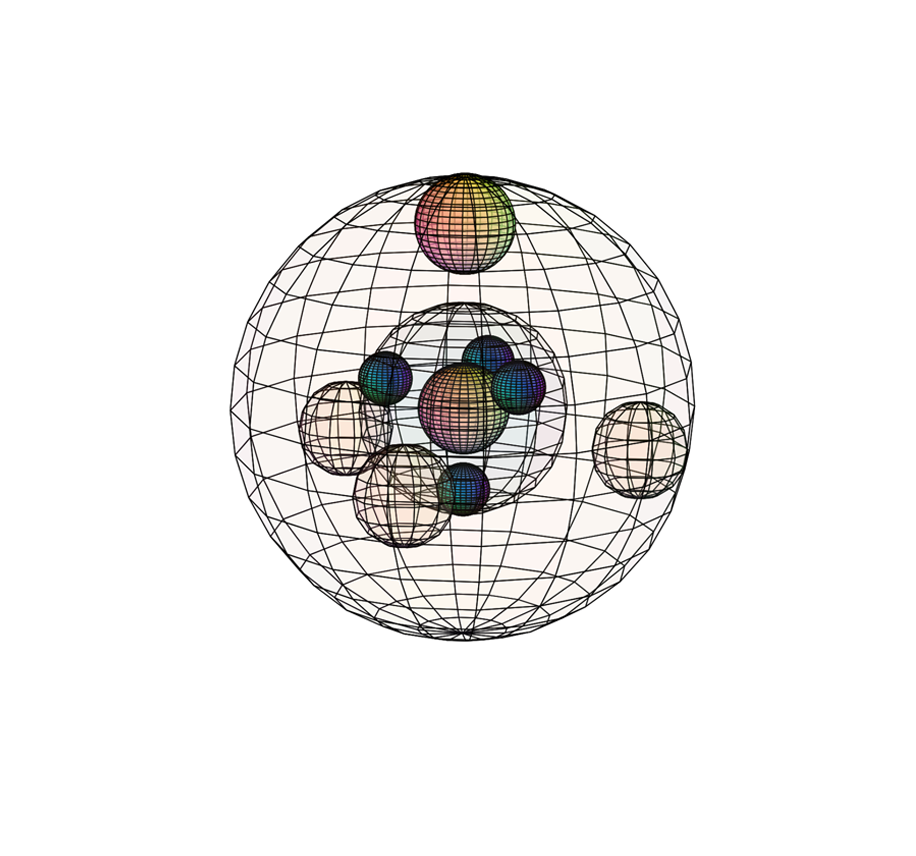
PHOSPHORUS (15P)
Phosphorus experiences a similar orbital hybridization to carbon, also resulting in a tetrahedral (4-directional) geometry. Its three unpaired valence electrons are able to form a tri-electron resonance structure on the same side of the atomic sphere. This greater concentration of electron density (than in silicon) gives phosphorus’s ‘multi-electron’ state a greater coherence, resulting in a much larger ionization energy (1,012 kJ/mol) than silicon (and even than sulfur). Phosphorus has a higher effective nuclear charge and a stronger inward attraction than in silicon, with a bonding atomic radius of 101 pm. Since it has a higher electronegativity (χe=2.19), phosphorus becomes more powerful at attracting electrons than silicon, though not as powerful as the smaller nitrogen atom in the 2nd period above it.
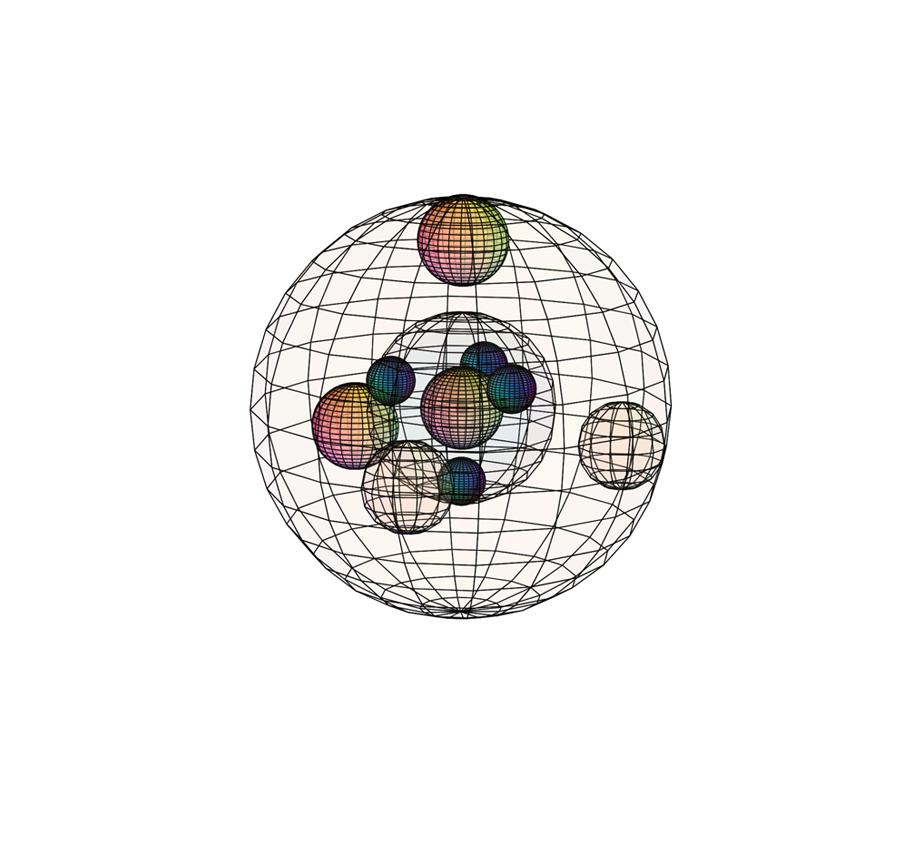
SULFUR (16S)
Sulfur experiences a similar orbital hybridization to carbon, also resulting in a tetrahedral (4-directional) geometry. Sulfur is only 2 electrons away from a full and stable 4-di-electron shell. As a result, it will attract electrons strongly, though not as strongly as oxygen in the 2nd period above it. This makes it less willing to release an electron, giving it a high ionization energy (1,000 kJ/mol), though not quite as high as phosphorus (due to its tri-electron coherence). Sulfur has a higher effective nuclear charge and a stronger inward attraction than in phosphorus, with a bonding atomic radius of 100 pm. Since it has a higher electronegativity (χe=2.58), sulfur becomes more powerful at attracting electrons, and can often make molecules polar as a result.
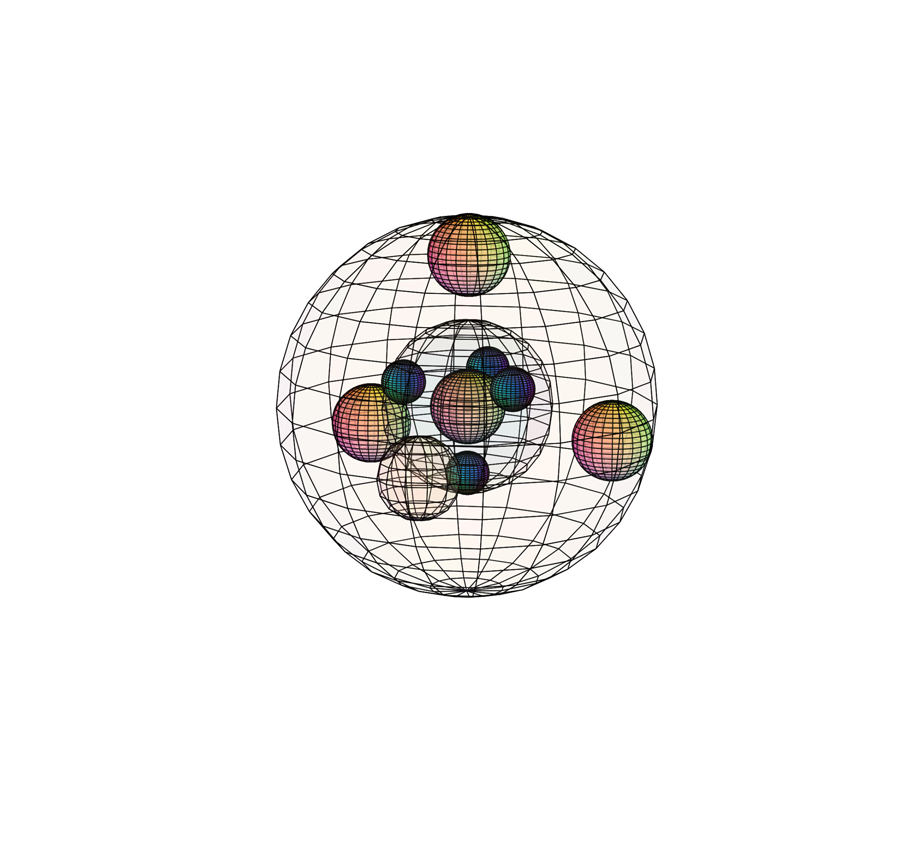
CHLORINE (17Cl)
Chlorine experiences a similar orbital hybridization to carbon, also resulting in a tetrahedral (4-directional) geometry. Chlorine is only 1 electron away from a full and stable 4-di-electron shell. As a result, it will attract electrons very strongly, though not quite as strongly as fluorine in the 2nd period above it. This makes it unwilling to release an electron, giving it a high ionization energy (1,251 kJ/mol). It has a higher effective nuclear charge and a stronger inward attraction than in sulfur, with a bonding atomic radius of 99 pm. Since it has a high electronegativity (χe=3.16), chlorine is very powerful at attracting electrons, and can therefore be quite reactive as it removes electrons from other substances.

ARGON (18Ar)
Argon experiences a similar orbital hybridization to carbon, also resulting in a tetrahedral (4-directional) geometry. It has achieved a full and stable 4-di-electron shell. As a result, it will neither attract electrons nor want any electron taken from it. This gives it a very high ionization energy (1,521 kJ/mol). It has a higher effective nuclear charge and a stronger inward attraction than chlorine. This makes it a smaller atom than chlorine, and the smallest on the second period. Since it makes no bonds, it has no measured bonding atomic radius and no electronegativity.
PERIODIC TRENDS: PERIOD II, PERIOD III, GROUP I, GROUP II, GROUP VII, GROUP VIII
RETURN to the Periodic Table
