
RETURN to Periodic Table
The second row (or period) of the periodic table is the first to feature atoms with electrons in a p-orbital resonance. This allows the electron geometry to expand from 1 spherical “direction” to 3- or 4-directional symmetries.

PERIODIC TRENDS:
Effective nuclear charge (Zeff) increases across the period.
Therefore:
Atomic radius decreases across the period.
Ionization energy increases across the period.
Electronegativity increases across the period
As we move across the second period, elements become smaller. This occurs because the next element in the row, while in the same shell, has one more proton and one more electron than the element to its left. This means there is a greater inward attraction between the more positive nucleus and the more negative electron cloud, which is called effective nuclear charge (Zeff), and this shrinks the size of the atom somewhat.

As a result of the increasing Zeff, each element in the period has a higher electronegativity than the element before it. The ionization energy also generally increases across the period, although there are exceptions. As we see in the graph below, which highlights the elements of this 2nd period, beryllium and nitrogen (in blue) have values that are larger than their neighbors. This is due to the added stability of their electron structures. We also see that ionization energies decrease as we proceed from one period to the next.

The first elements in the row are most willing to lose their electrons to achieve a full 1st electron shell, but as we proceed further along and the atoms gain electrons in the 2nd shell, they increasingly want to gain electrons to fill the 2nd shell.

LITHIUM (3Li)
Being in Group I, lithium is an alkali metal. With only one electron in its 2s orbital, lithium is much more willing to lose its single valence electron, since it would then have a full 1st shell — a stable 1s2 di-electron. This shows that a full electron shell is more stable than a neutral charge. Since lithium is a relatively small atom, its nuclear attraction is rather strong. Lithium is therefore only mildly reactive, bubbling as it gives off hydrogen gas when placed in water. As the first metal on the periodic table, it has a much lower electronegativity (χe=0.98) and ionization energy (520 kJ/mol) than the non-metal hydrogen (1H) atom above it. With only 3 nuclear protons attracting 3 electrons, lithium has a bonding atomic radius of 145 pm, and is the largest atom in the second period. It is paramagnetic (χm=+14.2), which means that it is attracted to a magnetic field because its unpaired outer electron can align with an external magnetic field.

BERYLLIUM (4Be)
Being in Group II, beryllium is an alkali earth metal. It has a stable 2s di-electron, and is therefore less reactive than lithium. It is also the least reactive of the Group II elements since it is the smallest of them. Beryllium has 4 nuclear protons attracting 4 electrons, giving it a higher effective nuclear charge and a stronger inward attraction. This makes it a smaller atom than lithium, with a bonding atomic radius of 105 pm, and gives it a higher electronegativity (χe=1.57). Beryllium has a much higher ionization energy (899 kJ/mol) than lithium due to the greater stability of its valence di-electron, which is harder to disrupt than an unpaired electron. Unlike lithium, beryllium is diamagnetic (χm=–9). This means that it repels a magnetic field because its valence di-electron is perfectly aligned, magnetically, and therefore resists an external magnetic field.

BORON (5B)
Boron is the first atom whose electrons experience orbital hybridization, resulting in a triangular (trigonal planar) geometry of unpaired electrons. As a result, its ionization energy (801 kJ/mol) is not quite as high as that of beryllium. Boron has 5 nuclear protons attracting 5 electrons, giving it a higher effective nuclear charge and a stronger inward attraction. This makes it a smaller atom than beryllium, with a bonding atomic radius of 80 pm, which gives it a higher electronegativity (χe=2.04), less reactivity, and a less metallic nature than beryllium. Boron is the first semi-metal (or metalloid), giving it unique properties as a result.

CARBON (6C)
Carbon is the second atom whose electrons experience orbital hybridization, resulting in a tetrahedral (4-directional) geometry of unpaired electrons. As a result, its ionization energy (1,086 kJ/mol) is higher than that of boron (and even than beryllium) since it has 4 electrons in a stable and spherically-symmetrical resonance structure. Carbon has 6 nuclear protons attracting 6 electrons, giving it a higher effective nuclear charge and a stronger inward attraction, with a bonding atomic radius of 75 pm. Since it has a higher electronegativity (χe=2.55) than boron, carbon is the first non-metal atom in the second period, giving it unique properties as a result.

NITROGEN (7N)
Nitrogen’s electrons experience a similar orbital hybridization to that of carbon, also resulting in a tetrahedral (4-directional) geometry. Nitrogen’s three unpaired valence electrons are able to form an effective tri-electron resonance structure on the same side of the atomic sphere. This greater concentration of electron density (than in carbon) gives nitrogen’s ‘multi-electron’ state a greater coherence, resulting in a much larger ionization energy (1,402 kJ/mol) than carbon (and even than oxygen). Nitrogen has 7 nuclear protons attracting 7 electrons, giving it a higher effective nuclear charge and a stronger inward attraction than in carbon, with a bonding atomic radius of 70 pm. Since it has a higher electronegativity (χe=3.04), nitrogen becomes much more powerful at attracting electrons, and will often make molecules polar as a result, as we see in ammonia molecule (NH3).

OXYGEN (8O)
Oxygen’s electrons experience a similar orbital hybridization to that of carbon, also resulting in a tetrahedral (4-directional) geometry. Oxygen is only 2 electrons away from a full and stable 4-di-electron shell. As a result, it will attract electrons strongly. This makes it far less willing to release an electron, giving it a high ionization energy (1,314 kJ/mol), though not quite as high as nitrogen (due to its tri-electron coherence). Oxygen has 8 nuclear protons attracting 8 electrons, giving it a higher effective nuclear charge and a stronger inward attraction than in nitrogen, with a bonding atomic radius of 65 pm. Since it has a higher electronegativity (χe=3.44), oxygen becomes much more powerful at attracting electrons, and will often make molecules polar as a result (as in water).
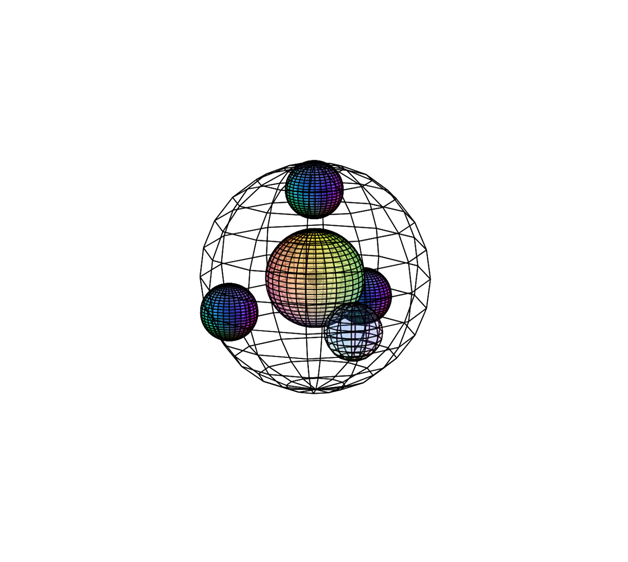
FLUORINE (9F)
Fluorine’s electrons experience a similar orbital hybridization to that of carbon, also resulting in a tetrahedral (4-directional) geometry. Fluorine is only 1 electron away from a full and stable 4-di-electron shell. As a result, it will attract electrons extremely strongly. This makes it unwilling to release an electron, giving it a very high ionization energy (1,681 kJ/mol). It has 9 nuclear protons attracting 9 electrons, giving it a higher effective nuclear charge and a stronger inward attraction than in oxygen, with a bonding atomic radius of 60 pm. Since it has the highest electronegativity (χe=3.98) on the periodic table, fluorine is extremely powerful at attracting electrons, and will often degrade other substances as it removes electrons from them.
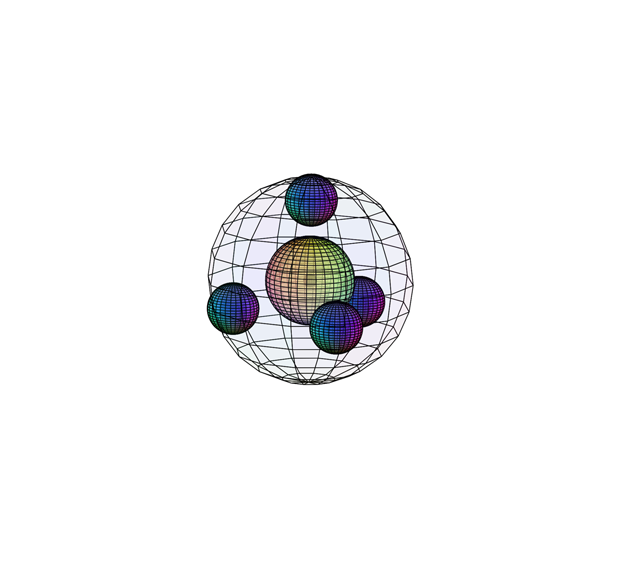
NEON (10Ne)
Neon’s electrons experience a similar orbital hybridization to that of carbon, also resulting in a tetrahedral (4-directional) geometry. Neon has achieved a full and stable 4-di-electron shell. As a result, it will neither attract electrons nor want any electron taken from it. This gives it a very high ionization energy (2,081 kJ/mol). It has 10 nuclear protons attracting 10 electrons, giving it a higher effective nuclear charge and a stronger inward attraction than fluorine. This makes it a smaller atom than fluorine, and the smallest on the second period. Since it makes no bonds, it has no measured bonding atomic radius and no electronegativity.
PERIODIC TRENDS: PERIOD II, PERIOD III, GROUP I, GROUP II, GROUP VII, GROUP VIII
RETURN to the Periodic Table
